| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
COX-2 (IC50 = 3.9 μM); COX-1 (IC50 = 22.3 μM); FAAH (IC50 = 78.6 μM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
化合物 1,或称卡洛芬,是一种非甾体类抗炎药。作为 FAAH/COX 的多靶点抑制剂,COX-2、COX 和 FAAH 的 IC50 值分别为 3.9 μM、22.3 μM 和 78.6 μM-1。在 CCL 和 CaCL 细胞中,卡洛芬 (10 μg/mL) 具有细胞保护特性并降低细胞凋亡。与相应的 CCL 或 CaCL 对照相比,卡洛芬 (10 μg/mL) 并未显着增加 PGE2 浓度 [2]。
NSAIDs的细胞保护作用取决于SNP诱导的凋亡程度,在具有中度SNP诱导的细胞毒性作用的CCL和CaCL细胞培养中最强。当CCL和CaCL细胞随后与SNP一起孵育时,用NSAID预孵育可将细胞存活率提高15%至45%。Carprofen(10μg/mL)对CCL和CaCL细胞具有最大的细胞保护作用。与非甾体抗炎药一起孵育导致SNP损伤细胞PGE(2)的产生没有显著减少。 结论和临床相关性:结果表明,卡罗芬、美洛昔康和罗布那昔布可能会减少犬交叉韧带细胞的凋亡[2] |
||
| 体内研究 (In Vivo) |
第 3 天和第 10 天,卡洛芬(2.2 mg/kg,口服)显着降低了犬血液中 PGE2 的水平。第三天,卡洛芬同样减少了胃中 PGE2 的合成;然而,到了第 10 天,抑制作用就没那么大了。此外,在第3天和第10天,证明卡洛芬对狗胃内PGE1的合成没有影响[3]。
|
||
| 酶活实验 |
体外试验[1]
在37°C[3H]anandamide(1 uM冷AEA和0.6 nM(1 mCi/mL)[3H]-AEA(花生四烯酸-[1-3H]乙醇胺,比活度60 Ci/mmol)下孵育30分钟,在测定缓冲液(50 mM TRIS pH 7.4,0.05%无脂肪酸BSA)中存在50 ug蛋白质/总大鼠脑匀浆样品的情况下,测量FAAH活性。用冷的1:1 CHCl3/MeOH停止反应。通过液体闪烁对水相进行计数(Microbeta2-Lumijet,改编自Kathuria等人,2003)。在添加底物之前,抑制剂与适当浓度的酶制剂预孵育10分钟。 使用商业酶免疫测定试剂盒测量COX活性。除底物浓度外,遵循制造商协议。简而言之,抑制剂与绵羊COX-1或人COX-2在37°C下预孵育10分钟,反应在5μM花生四烯酸存在下在37°C下进行2分钟。用盐酸停止反应,然后用SnCl2将COX衍生的PGH2转化为PGF2α。然后使用PG特异性抗体通过酶免疫测定(EIA)定量PGF2α产物,并与PG乙酰胆碱酯酶偶联物竞争。使用帝肯Infinite M200板读数器在412 nM下测量吸光度,并根据制造商的说明处理数据[1]。 |
||
| 细胞实验 |
CCL和CaCL细胞的原代培养是通过十字韧带外植体的酶解产生的。纯化的细胞培养物在没有(对照)或有3种浓度的4种非甾体抗炎药中的1种(10、100或200μg乙酰水杨酸/mL;0.1、1或10μg卡罗芬/mL;0.1,1或10μg/mL美洛昔康/mL;或0.1,1、10μg罗贝昔b/mL)的情况下孵育2小时,随后与3种浓度SNP中的1个孵育18小时,以诱导轻度、中度或重度细胞毒性作用。分别通过细胞增殖试验和流式细胞术分析细胞活力和凋亡。通过ELISA测定前列腺素E(2)浓度[2]。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Rapidly and nearly completely absorbed (more than 90% bioavailable) when administered orally. Metabolism / Metabolites Hepatic. Biological Half-Life Approximately 8 hours (range 4.5–9.8 hours) in dogs. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
High (99%) |
||
| 参考文献 |
|
||
| 其他信息 |
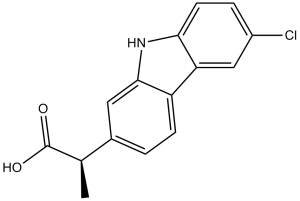
Carprofen is propanoic acid in which one of the methylene hydrogens is substituted by a 6-chloro-9H-carbazol-2-yl group. A non-steroidal anti-inflammatory drug, it is no longer used in human medicine but is still used for treatment of arthritis in elderly dogs. It has a role as a non-steroidal anti-inflammatory drug, an EC 1.14.99.1 (prostaglandin-endoperoxide synthase) inhibitor and a photosensitizing agent. It is a member of carbazoles and an organochlorine compound.
Carprofen is a non-steroidal anti-inflammatory drug (NSAID) that is used by veterinarians as a supportive treatment for the relief of arthritic symptoms in geriatric dogs. Carprofen was previously used in human medicine for over 10 years (1985-1995). It was generally well tolerated, with the majority of adverse effects being mild, such as gastro-intestinal pain and nausea, similar to those recorded with aspirin and other non-steroidal anti-inflammatory drugs. It is no longer marketed for human usage, after being withdrawn on commercial grounds. Carprofen is a propionic acid derivate and nonsteroidal anti-inflammatory drug (NSAID) with anti-inflammatory, analgesic, and antipyretic activities, used exclusively in veterinary medicine. Carprofen inhibits the activity of the enzymes cyclo-oxygenase (COX) I and II, resulting in a decreased formation of precursors of prostaglandins and thromboxanes. This inhibits the formation of prostaglandins, by prostaglandin synthase, that are involved in pain, inflammation and fever. Ibuprofen also causes a decrease in the formation of thromboxane A2 synthesis, by thromboxane synthase, thereby inhibiting platelet aggregation. Drug Indication For use as a pain reliever in the treatment of joint pain and post-surgical pain. Mechanism of Action The mechanism of action of carprofen, like that of other NSAIDs, is believed to be associated with the inhibition of cyclooxygenase activity. Two unique cyclooxygenases have been described in mammals. The constitutive cyclooxygenase, COX-1, synthesizes prostaglandins necessary for normal gastrointestinal and renal function. The inducible cyclooxygenase, COX-2, generates prostaglandins involved in inflammation. Inhibition of COX-1 is thought to be associated with gastrointestinal and renal toxicity while inhibition of COX-2 provides anti-inflammatory activity. In an in vitro study using canine cell cultures, carprofen demonstrated selective inhibition of COX-2 versus COX-1. Pharmacodynamics Carprofen is a non-steroidal anti-inflammatory drug (NSAID) of the propionic acid class that includes ibuprofen, naproxen, and ketoprofen. It is no longer used in the clinical setting, but is approved for use in dogs. Carprofen is non-narcotic and has characteristic analgesic and antipyretic activity approximately equipotent to indomethacin in animal models. |
| 分子式 |
C15H12CLNO2
|
|
|---|---|---|
| 分子量 |
273.71
|
|
| 精确质量 |
273.055
|
|
| 元素分析 |
C, 65.82; H, 4.42; Cl, 12.95; N, 5.12; O, 11.69
|
|
| CAS号 |
53716-49-7
|
|
| 相关CAS号 |
Carprofen-d3;1173019-42-5;Carprofen-13C,d3;2012598-34-2
|
|
| PubChem CID |
2581
|
|
| 外观&性状 |
Typically exists as White to off-white solids at room temperature
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
509.1±35.0 °C at 760 mmHg
|
|
| 熔点 |
186-188ºC
|
|
| 闪点 |
261.7±25.9 °C
|
|
| 蒸汽压 |
0.0±1.4 mmHg at 25°C
|
|
| 折射率 |
1.732
|
|
| LogP |
4.03
|
|
| tPSA |
53.09
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
2
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
19
|
|
| 分子复杂度/Complexity |
362
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
ClC1C([H])=C([H])C2=C(C=1[H])C1C([H])=C([H])C(=C([H])C=1N2[H])C([H])(C(=O)O[H])C([H])([H])[H]
|
|
| InChi Key |
PUXBGTOOZJQSKH-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C15H12ClNO2/c1-8(15(18)19)9-2-4-11-12-7-10(16)3-5-13(12)17-14(11)6-9/h2-8,17H,1H3,(H,18,19)
|
|
| 化学名 |
2-(6-chloro-9H-carbazol-2-yl)propanoic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (7.60 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (7.60 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (7.60 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.6535 mL | 18.2675 mL | 36.5350 mL | |
| 5 mM | 0.7307 mL | 3.6535 mL | 7.3070 mL | |
| 10 mM | 0.3654 mL | 1.8268 mL | 3.6535 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06420323 | Not yet recruiting | Device: Treatment with NovoX® Cup Device: Treatment with Omnistrip® |
Wound Healing Disorder Post-Surgical Complication Mammaplasty |
MOSS S.p.A. | June 2024 | |
| NCT06458478 | Recruiting | Other: Hyper-oxygenated gel Other: glycerin based gel |
Molar, Fourth Extracting Own Teeth Edema Face (and 2 more...) |
Azienda Ospedaliera di Perugia | July 1, 2024 | Not Applicable |
| NCT03911336 | Withdrawn | Drug: Group A - test:Tooth extraction and intake of NSAID and a non-NSAID Drug: Group B - Control 1: tooth extraction and intake of NSAID and a non-NSAID Drug: Group C - Control 2: tooth extraction and intake of a Non-Nsaid |
Tooth Loss | University of Iowa | January 1, 2023 | Phase 4 |
| NCT01448785 | Unknown † | Device: abiliti system implant Device: Laparoscopic adjustable gastric band (Allergan Lap Band) |
Obesity Morbid Obesity |
IntraPace, Inc | April 2011 | Not Applicable |
|
|
|