| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 靶点 |
Cathepsin B; neuroprotective, anti-cancer, and anti-inflamatory
|
|---|---|
| 体外研究 (In Vitro) |
CA-074Me(5 μM 和 50 μM)可抑制来自 C57BL/6J 和 NOD/ShiLtJ 小鼠的 BMM 细胞中 RANKL 诱导的破骨细胞生成。在体外 RANKL 刺激后 24 小时观察 CA-074Me 的抗破骨细胞活性。值得注意的是,这种影响不是由 MAPK-ERK 信号级联介导的。钠诱导的 NFATc1 自身增强和 RANKL 刺激的 c-FOS 上调均被 CA-074Me 抑制 [2]。 CA-074Me 会抑制 CVB1 诱导的细胞植入 [3]。
|
| 体内研究 (In Vivo) |
治疗前后,CA074-me(1 μg、10 μg)可保护海马 CA1 神经元免受全脑 I/R 损伤诱导的程序化甲苯的影响。 CA074-me 显着抑制组织蛋白酶-B 渗漏和溶酶体膜破裂。除了抑制 RIP3 的过度表达和核转位外,CA074-me 还可以增加 I/R 损伤后 Hsp70 的上调并降低 NAD+ 水平 [1]。与CVB+无组相比,接受CA-074Me(4mg/kg/天,肌肉注射)的豚鼠组具有显着更高的中心评分。 30 mg/kg)可防止骨质恶化和破骨细胞生成[2]。 CVB+CA-074Me组的CD8+T细胞比假手术组少[3]。
|
| 酶活实验 |
氧-葡萄糖剥夺(OGD)和ddn -153染色[1]
OGD在无葡萄糖脱氧缓冲介质中进行,在OGD室中,95% N2和5% CO2气氛,37℃下,OGD 2 h。假手术组放置在含有25 mM葡萄糖的类似缓冲液中,在培养箱中保存2 h。CA074-me组于OGD暴露前1 h,在含有1 μg CA074-me的培养基中孵育。采用LysoSensor Green DND-153染色观察OGD损伤22 h后海马神经元溶酶体pH的变化。细胞在含1 μM LysoSensor Green DND-153的培养基中孵育60分钟,并用该培养基洗涤数次。然后在倒置荧光显微镜或共聚焦显微镜下观察组织。 抗酒石酸酸性磷酸酶(TRAP)测定[2] 根据制造商的说明,使用酸性磷酸酶染色试剂盒进行TRAP检测。细胞培养和破骨细胞生成7天后,取出培养基并用PBS洗涤三次。用制造商提供的固定溶液固定mm。细胞在37℃下与含有去离子水、Fast Garnet GBC、磷酸萘酚、醋酸酯和酒石酸盐的溶液孵育1小时。取下染色液,用PBS(3倍)洗涤,风干。光镜下,整个培养区有三个或更多核的TRAP阳性细胞计数为多核破骨细胞[2]. 颅组织切片的TRAP染色方法与此类似。用PBS(3倍)洗涤载玻片,并在含有去离子水、Fast Garnet GBC、磷酸萘酚、醋酸盐和酒石酸盐(TRAP试剂盒)的溶液中于37℃孵育1小时。载玻片用PBS(3倍)洗涤,风干,用水性贴载介质贴载。光镜下观察颅骨缝合区有3个或更多核的TRAP阳性细胞为多核破骨细胞。 |
| 细胞实验 |
免疫印迹分析[2]
细胞接种于6孔板,密度为0.2 × 106个/孔,培养条件与“小鼠骨髓巨噬细胞分离、培养和破骨细胞形成”章节相同。用RANKL和/或CA074-me刺激细胞,并用冷PBS洗涤2次。用RIPA裂解缓冲液收集细胞并进行超声处理。用8% SDS-PAGE分离20 μg蛋白裂解液,转移到硝化纤维素膜上。将膜用5%脱脂乳在含Tween-20的Tris- buffered Saline (TBST)中(25mM Tris/HCl, pH7.6, 150 mM NaCl, 0.1% Tween-20)在室温下阻断1小时,然后与包括NFATc1, pERK1/2, ERK1/2, c-FOS和GAPDH (Cell Signaling)在内的一抗孵育过夜42然后用TBST(3倍洗涤10分钟)洗涤膜,用辣根过氧化物酶(HRP)偶联二抗孵育30分钟。使用商用酶标检测试剂将蛋白质可视化。 免疫细胞化学[2] 在8室载玻片中,加入30 ng/ml M-CSF的MEMα培养基培养BMMs 3天。之后,分别用30 ng/ml M-CSF、50ng/ml RANKL,含或不含50 μM CA074-me刺激bmm 15 min。细胞固定在4%多聚甲醛磷酸盐缓冲液中,0.1% triton -100渗透,然后用NF-κB p65一抗(Abcam)染色。二抗选用Alexa Fluor 488兔抗小鼠IgG。 |
| 动物实验 |
In Vivo RANKL-Induced Osteoclastogenesis[2]
All animal procedures were performed in accordance with approved IACUC protocols. RANKL (0.08 mg/kg) with and without CA074-me (10 mg/kg or 30 mg/kg) were mixed in sterile, nonimmunogenic 1% Extracel-HP gel according to the manufacturer's instructions. The gel is composed of thiol-modified sodium hyaluronate, thiol-modified heparin, thiol-modified gelatin, and degassed deionized sterile water. The hydrogel mixture was prepared in an aseptic hood using a sterile syringe. The control sham hydrogel contained sterile Phosphate Buffered Saline (PBS) without any cytokines. The osteolysis group was given 0.08 mg/kg RANKL in a hydrogel to induce pathologic bone loss, as described in previously published papers.6, 16 The hydrogel-only, hydrogel-RANKL, and hydrogel-RANKL-CA-074Me mixture was injected into 8-week old male mice calvarium in an aseptic hood (n = 5) following general anesthesia (80 mg/kg of ketamine and 7 mg/kg of xylazine). After four days, the calvaria were excised, fixed in 4% formaldehyde for 24 h, decalcified in 20% EDTA for one week, and sectioned into slides from paraffin blocks. The slides underwent Tartrate-Resistant Acid Phosphatase (TRAP) staining (see section Tartrate Resistant Acid Phosphatase (TRAP) Assay) to identify osteoclasts. lobal cerebral I/R injury model and drug administration[1] Four-vessel occlusion (4-VO) for global cerebral ischemia with minor modification as described in our previous study was used (Wang et al., 2011). Under 4% (w/v) choral hydrate (400 mg/kg) anesthesia, both vertebral arteries were permanently electro-cauterized and the bilateral common carotid arteries (CCAs) were freed from surrounding tissues. After closing the surgical incisions, rats were allowed to recover for 24 h. On the following day, anesthesia was induced with 4% isoflurane and the CCAs were occluded with aneurysm clips for 20-min to induce global cerebral ischemia, then the clips were removed for reperfusion. Rectal temperature was maintained at 37 ± 0.5 °C throughout the procedures. Rats were moved to the animal’s incubator to keep the proper temperature until fully awake. Rats with dilated pupils and without seizures were selected for experiments.[2] CA074-me was dissolved in 0.9% NaCl containing 2% DMSO to 0.2 μg/μl or 2 μg/μl. 1 h before ischemia or 1 h post reperfusion, CA074-me (0.2 μg/μl or 2 μg/μl) or vehicle (2% DMSO) was injected into the right cerebral ventricle (anteroposterior −0.92; mediolateral 1.5; dorsoventral 3.5 mm) with a total volume of 5 μl at 0.5 μl/min. Rats were assigned to 5 groups: the I/R group received 5 μl vehicle; three CA074-me groups were subjected to the same procedures as the I/R group, and received 1 μg CA074-me (CA074-me 1 μg) or 10 μg CA074-me (CA074-me 10 μg) 1 h before 20-min ischemia and 1 μg CCA074-me 1 h post reperfusion (Post CA074-me 1 μg) respectively; the sham group was subjected to the same procedures as the I/R group, except for occlusion of the CCAs. Thirty-two female shorthair guinea pigs (body weight: 154±18 g and age: 4 weeks old) were used. The animal models of PM were established by injection with CVB1 as described previously.16 In brief, four experimental groups(each group contains eight guinea pigs) were included: (A) sham group: received normal saline; (B) CVB1+ None group: received CVB1+Freunds complete adjuvant (FCA); (C) CVB1+ Saline group (pseudo-intervention group): received CVB1+FCA+normal saline (NS); and (D) CVB1+CA074-me group: CVB1+FCA+CA-074Me. CA074-me (4 mg/kg/day i.m.) was given 24 h after CVB1 injection for 7 consecutive days. Four weeks after CVB1 injection, the animals were killed, and lung tissues were collected for the following experiments.[3] |
| 参考文献 |
|
| 其他信息 |
CA-074 methyl ester (CA-074Me) is a cell-permeable cathepsin B inhibitor. It is converted by cellular esterases to CA-074.
Many studies have demonstrated the key role of lysosomes in ischemic cell death in the brain and have led to the "lysosomocentric" hypothesis. In this hypothesis, the release of cathepsin-B due to a change of lysosomal membrane permeabilization (LMP) or rupture is critical, and this can be prevented by its inhibitors CA074 and CA074-me. However, the role of CA074-me in neuronal death and its effect on the change of lysosomal membrane integrity after global cerebral ischemia/reperfusion (I/R) injury is not clear, so we investigated this here. Rat hippocampal CA1 neuronal death was evaluated after 20-min global cerebral I/R injury. CA074-me (1 μg, 10 μg) were given intracerebroventricularly 1h before ischemia or 1h post reperfusion. The changes of heat shock protein 70 (Hsp70), cathepsin-B, lysosomal-associated membrane protein 1 (LAMP-1), receptor-interacting protein 3 (RIP3), and the change of lysosomal pH were evaluated respectively. Hippocampal CA1 neuronal programmed necrosis induced by global cerebral I/R injury was prevented by CA074-me both pre-treatment and post-treatment. Diffuse cytoplasmic cathepsin-B and LAMP-1 immunostaining synchronized with the pyknotic nuclear changes 2 days post reperfusion, and a rise of lysosomal pH with the leakage of DND-153, a dye of lysosomes, after oxygen-glucose deprivation (OGD) was detected. Both of these changes demonstrated the rupture of lysosomal membrane and the leakage of cathepsin-B, and this was strongly inhibited by CA074-me pre-treatment. The overexpression and nuclear translocation of RIP3 and the reduction of NAD(+) level after I/R injury were also inhibited, while the upregulation of Hsp70 was strengthened by CA074-me pre-treatment. Delayed fulminant leakage of cathepsin-B due to lysosomal rupture is a critical harmful factor in neuronal programmed necrosis induced by 20-min global I/R injury. In addition to being an inhibitor of cathepsin-B, CA074-me may have an indirect neuroprotective effect by maintaining lysosomal membrane integrity and protecting against lysosomal rupture.[1] The osteoclast is an integral cell of bone resorption. Since osteolytic disorders hinge on the function and dysfunction of the osteoclast, understanding osteoclast biology is fundamental to designing new therapies that curb osteolytic disorders. The identification and study of lysosomal proteases, such as cathepsins, have shed light on mechanisms of bone resorption. For example, Cathepsin K has already been identified as a collagen degradation protease produced by mature osteoclasts with high activity in the acidic osteoclast resorption pits. Delving into the mechanisms of cathepsins and other osteoclast related compounds provides new targets to explore in osteoclast biology. Through our anti-osteoclastogenic compound screening experiments we encountered a modified version of the Cathepsin B inhibitor CA-074: the cell membrane-permeable CA-074Me (L-3-trans-(Propylcarbamoyl) oxirane-2-carbonyl]-L-isoleucyl-L-proline Methyl Ester). Here we confirm that CA-074Me inhibits osteoclastogenesis in vivo and in vitro in a dose-dependent manner. However, Cathepsin B knockout mice exhibited unaltered osteoclastogenesis, suggesting a more complicated mechanism of action than Cathepsin B inhibition. We found that CA-074Me exerts its osteoclastogenic effect within 24 h of osteoclastogenesis stimulation by suppression of c-FOS and NFATc1 pathways.[2] Cathepsin B (CB) is involved in the turnover of proteins and has various roles in maintaining the normal metabolism of cells. In our recent study, CB is increased in the muscles of polymyositis/dermatomyositis (PM/DM). However, the role of CB in interstitial lung disease (ILD) has not been reported. ILD is a frequent complication of PM/DM, which is the leading cause of death in PM/DM. It carries high morbidity and mortality in connective tissue diseases, characterized by an overproduction of inflammatory cytokines and induced fibrosis, resulting in respiratory failure. The etiology and pathogenesis of ILD remain incompletely understood. This study investigated whether treatment with CA-074Me, a specific inhibitor of CB, attenuates ILD in PM. CB expression, inflammation, and fibrosis were analyzed in the lung tissues from patients with PM/DM. The animal model of PM was induced in guinea pigs with Coxsackie virus B1 (CVB1). CA-074Me was given 24 h after CVB1 injection for 7 consecutive days. At the end of the experiment, the animals were killed and lung tissues were collected for the following analysis. Inflammation, fibrosis and apoptosis cells, and cytokines were assessed by histological examinations and immunohistochemical analyses, western blot analysis and transferase-mediated dUTP nick-end labeling assay. In patients with PM/DM, the protein levels of CB were significantly elevated in lung tissues compared with healthy controls, which correlated with increases in inflammation and fibrosis. Similarly, the expression of CB, inflammation and fibrosis, CD8(+) T cell, CD68(+) cell, tumor necrosis factor-alpha, transforming growth factor-beta1 infiltrations, and apoptotic cell death were significantly increased in lung tissues of the guinea-pig model of CVB1-induced PM. These changes were attenuated by the administration of CA-074Me. In conclusion, this study demonstrates that PM/DM increases CB expression in lung tissues and inhibition of CB reduces ILD in a guinea-pig model of CVB1-induced PM. This finding suggests that CB may be a potential therapeutic target for ILD.[3] |
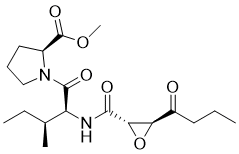
| 分子式 |
C19H30N2O6
|
|---|---|
| 分子量 |
382.45
|
| 精确质量 |
397.221
|
| 元素分析 |
C, 59.67; H, 7.91; N, 7.32; O, 25.10
|
| CAS号 |
147859-80-1
|
| 相关CAS号 |
134448-10-5
|
| PubChem CID |
6610318
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
0.694
|
| tPSA |
117.34
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
611
|
| 定义原子立体中心数目 |
5
|
| SMILES |
O1[C@@]([H])(C(N([H])C([H])([H])C([H])([H])C([H])([H])[H])=O)[C@@]1([H])C(N([H])[C@]([H])(C(N1C([H])([H])C([H])([H])C([H])([H])[C@@]1([H])C(=O)OC([H])([H])[H])=O)[C@@]([H])(C([H])([H])[H])C([H])([H])C([H])([H])[H])=O
|
| InChi Key |
XGWSRLSPWIEMLQ-YTFOTSKYSA-N
|
| InChi Code |
InChI=1S/C19H31N3O6/c1-5-9-20-16(23)14-15(28-14)17(24)21-13(11(3)6-2)18(25)22-10-7-8-12(22)19(26)27-4/h11-15H,5-10H2,1-4H3,(H,20,23)(H,21,24)/t11-,12-,13-,14-,15-/m0/s1
|
| 化学名 |
methyl (2S)-1-[(2S,3S)-3-methyl-2-[[(2S,3S)-3-(propylcarbamoyl)oxirane-2-carbonyl]amino]pentanoyl]pyrrolidine-2-carboxylate
|
| 别名 |
Ca-074Me; Ca-074Me; CA-074 methyl ester; CA-074Me; Cathepsin B Inhibitor IV; methyl ((2S,3S)-3-(propylcarbamoyl)oxirane-2-carbonyl)-L-isoleucyl-L-prolinate; (S)-methyl 1-((2S,3S)-3-methyl-2-((2S,3S)-3-(propylcarbamoyl)oxirane-2-carboxamido)pentanoyl)pyrrolidine-2-carboxylate; methyl (2S)-1-[(2S,3S)-3-methyl-2-[[(2S,3S)-3-(propylcarbamoyl)oxirane-2-carbonyl]amino]pentanoyl]pyrrolidine-2-carboxylate; CA-074-Me; Ca-074Me
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~251.59 mM)
H2O : < 0.1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.29 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.29 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.29 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6147 mL | 13.0736 mL | 26.1472 mL | |
| 5 mM | 0.5229 mL | 2.6147 mL | 5.2294 mL | |
| 10 mM | 0.2615 mL | 1.3074 mL | 2.6147 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。