| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Toxicokinetic studies performed on mice, rats and rabbits indicate that bentazone is rapidly and almost completely absorbed via the oral route (> 99%), and maximum blood concentrations of radioactivity are achieved in approximately 15 minutes at low doses (4 mg/kg bw) and by 1 hour at high doses (200 mg/kg bw). Administration of bentazone either as the sodium salt or as the free acid did not result in any significant differences in absorption. There was no evidence of penetration into the central nervous system or spinal cord, and elimination from other tissues was rapid, with no indication of bioaccumulation. Elimination was almost exclusively via the urine (approximately 91% within 24 hours); 5 days after dosing, less than 2% was found in feces and less than 0.02% in expired air. Biliary excretion of radioactivity was minimal. No significant differences were found in absorption and elimination among the different species investigated (rat, rabbit, mouse). The dermal penetration of [14C]bentazone sodium salt (batch no. 210-2201, radiochemical purity 97.3%) through human skin was assessed by a single topical application of about 4933, 49.3 or 8.22 ug/sq cm of active ingredient formulated in BAS 351 32 H to split thickness skin membranes mounted on Franz-type diffusion cells. The doses represent the formulation concentrate or two representative spray dilutions (1:100 and 1:600) for field use, respectively. The study was performed using five diffusion cells per dose. ... It can be concluded that in vitro dermal penetration of bentazone formulated as an aqueous soluble (liquid) concentrate formulation of bentazone sodium through human skin is appropriately calculated as per cent absorbed dose. Considering the amount of radiolabeled substance associated with the skin (remaining skin and tape strips 3-6) after washing as absorbable and combining this with the absorbed amount detected in the receptor, the extent of dermal penetration through human epidermis is about 0.06% for the concentrate, 1.31% for the 1:100 spray strength dilution and 1.23% for the 1:600 dilution. /Bentazone sodium salt/ A case of fatal suicidal bentazone poisoning was presented along with a description of the different analytical methods involved. A 56-year-old farmer was examined by the family doctor 1 hour after voluntarily ingesting 500 mL of FIGHTER (about 250 g bentazone). He presented a Glasgow score of 15, polypnea, diarrhea and vomiting. During transport by ambulance to the hospital, he tossed, sweated and suddenly presented breathing difficulty followed by heart failure. The patient died within 2 hours post-ingestion. Blood and urine samples were taken just before death. Bentazone plasma and urine levels were 1500 and 1000 mg/L, respectively. A 59-year-old woman who intentionally ingested 100-200 mL Basagran (about 50-100 g bentazone) was taken to the hospital with cardiac arrest 2 days after she had consumed the herbicide. During this period, she suffered vomiting, urination and diarrhoea, and she was drowsy with a muddled speech. Biological samples obtained at the autopsy were analysed, and the presence of bentazone, alcohol and an active metabolite of citalopram was detected. Blood concentrations of bentazone, alcohol and desmethyl-citalopram were 625 mg/kg, 0.62 g/L and 0.03 mg/kg, respectively. Metabolism / Metabolites The metabolism of bentazone was investigated in a number of toxicokinetic studies following oral (rat and rabbit) or intravenous administration (mouse) ... . Bentazone was only poorly metabolized, with the parent compound being the predominant excretion product. Only small amounts of 6-hydroxybentazone and 8-hydroxybentazone could be detected. In rats, rabbits and mice, no conjugated products were found. 6-Hydroxybentazone and 8-hydroxybentazone are major plant metabolites of bentazone. Because crops of treated plants can be consumed by humans, farm animals or pets, an exposure to both of these compounds might be expected in principle. Although both metabolites have been demonstrated to be formed in mammals and therefore can be regarded as included in toxicological testing of the parent compound, specific toxicological studies were performed. It has been shown that the 8-hydroxy and 6-hydroxy metabolites of bentazone are of comparable toxicity by the oral route of administration and are both less toxic than the parent compound. Additionally, both metabolites were negative in the Ames assay for the potential to induce point mutations in bacteria. As it is unlikely that a hydroxy group shift in the bentazone ring system dramatically changes the toxicity, it was decided to perform further investigations on 8-hydroxybentazone as a reference substance. Therefore, 8-hydroxybentazone was investigated in a subchronic feeding study, in several mutagenicity studies and in a prenatal developmental study. These investigations revealed that the metabolites have no mutagenic or teratogenic potential and are less toxic than the parent substance. In studies with soybeans [Glycine max (Leguminatae) Merr.] and navy beans (Phaseolus vulgaris Leguminatae), four unidentified conjugates were observed. After foliar or root absorption, bentazon was rapidly metabolized by soybeans with hydroxylation at the 6 and 8 position. These were conjugated. Analysis of soybean field samples showed hydroxylation of bentazon in early growth stages. Although absorption and translocation of bentazon was not markedly different in resistant rice and susceptible C. serotinus, metabolism differed markedly. In rice, there was 80% metabolism of absorbed bentazon within 24 hr and 85% conversion to a major water soluble metabolite within 7 days. In C. serotinus, there was only 25-50% metabolism of bentazon in 7 days. Similar results were obtained with other resistant and susceptible plant species indicating that ability to metabolize this compound is the primary mechanism of selectivity. The primary metabolite in rice was identified by GC-MS, NMR and IR as 6-(bentazon)-O-beta-glucopyranoside. Other studies showed that the 6- and 8-hydroxybentazon were formed in about equal amounts in soybeans and that the 6-hydroxy analog predominates in wheat, rice, peanuts, Senecio sp., and Chenopodium sp. For more Metabolism/Metabolites (Complete) data for Bentazon (8 total), please visit the HSDB record page. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Bentazone is a white, crystalline solid. It was formerly used as an herbicide. HUMAN STUDIES: Bentazone is irritating to eyes and mucous membranes. A 50-year-old male who had sprayed corn with a solution of bentazone was admitted to the hospital with sweating, fever, nausea, vomiting of aqueous and hemorrhagic content, and bloody, watery stools. He was treated according to the symptoms including extracorporeal hemodialysis, but eventually suffered from multiorgan failure (acute respiratory failure, acute liver failure, coagulopathy, acute renal failure, metabolic acidosis, and gastrointestinal bleeding) and died 11.35 hr after admittance. In another case, intentional poisoning with 130 g of bentazone resulted in vomiting, fever, sweating, pipe-like muscle rigidity, sinus tachycardia, drowsiness, leukocytosis, rhabdomyolysis and hepatorenal damage. ANIMAL STUDIES: Bentazone is not a skin irritant but was a moderate eye irritant in rabbits. It is a skin sensitizer in guinea-pigs. In a chronic toxicity study, bentazone was administered to rats (50 of each sex per group) via a diet at doses 0, 5, 17 and 76 mg/kg bw per day for 2 years. Statistical analysis of tumor incidence did not reveal any significance among the groups tested. Bentazone was not teratogenic in rabbits or rats. In rat developmental studies, it increased post-implantation loss, skeletal variations (incomplete or absent ossification in the phalangeal nuclei of the extremities, sternebrae and cervical vertebrae) and reduced body weights of fetuses surviving to day 21 at 250 mg/kg bw per day. Dietary administration of bentazone to rats at dose levels of 0, 300, 1000 and 3500 ppm did not result in any indication of neurotoxicity. In vitro genotoxicity studies included bacterial reverse mutation assays on Salmonella typhimurium and Escherichia coli, DNA damage and repair studies on E. coli and Saccharomyces cerevisiae, and chromosomal aberration and forward mutation assays in CHO cells. In vivo studies included chromosome analyses in mice and rats, unscheduled DNA synthesis tests in mice, and mutation assays in germ cells for mice and rats. Bentazone gave negative results in all of these studies. ECOTOXICITY STUDIES: Bentazone affected zebrafish embryos and associated bacterial communities. It was nontoxic to bees. Toxicity Data LC50 (rat) = 5,100 mg/m3/4h Interactions Maize black Mexican sweet cell suspension cultures were used to study the effects of various cytochrome p450 monooxygenase inhibitors on the uptake and metabolism of the herbicide bentazon. Maize cells rapidly absorbed bentazon and metabolized it via aryl hydroxylation and glycosylation to a glycosyl conjugate of 6-hydroxybentazon. Maize black Mexican sweet cells accumulated bentazon to levels approximately 20 fold greater than those in the external medium. When maize black Mexican sweet cells were incubated in an external medium containing 25 uM bentazon, the formation of the glycosyl conjugate (ca 2 nmol/min/g fresh wt) was rate limited by aryl hydroxylation. Tetcyclacis, a plant growth retardant, phenylhydrazine, a mechanism based cytochrome p450 inhibitor, and piperonyl butoxide, an insecticide synergist, inhibited bentazon metabolism with I50 values of approximately 0.1, 1.0, and 1.0 uM, respectively. Other mechanism based cytochrome p450 inhibitors, 3(2,4-dichlorophenoxy)-1-propyne and aminobenzotriazole, also inhibited bentazon metabolism but were less effective. The results obtained with selected inhibitors are consistent with the hypothesis that aryl hydroxylation of bentazon is catalyzed by a cytochrome p450 monooxygenase. Non-Human Toxicity Values LD50 Rat oral 850-2470 mg/kg bw /Includes free acid and sodium salt forms; From table/ LD50 Guinea pig oral 1100 mg/kg bw /Free acid and sodium salt forms; From table/ LD50 Rabbit oral 1139 mg/kg bw /From table/ LD50 Rat dermal >5000 mg/kg bw /Acid form; From table/ For more Non-Human Toxicity Values (Complete) data for Bentazon (26 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
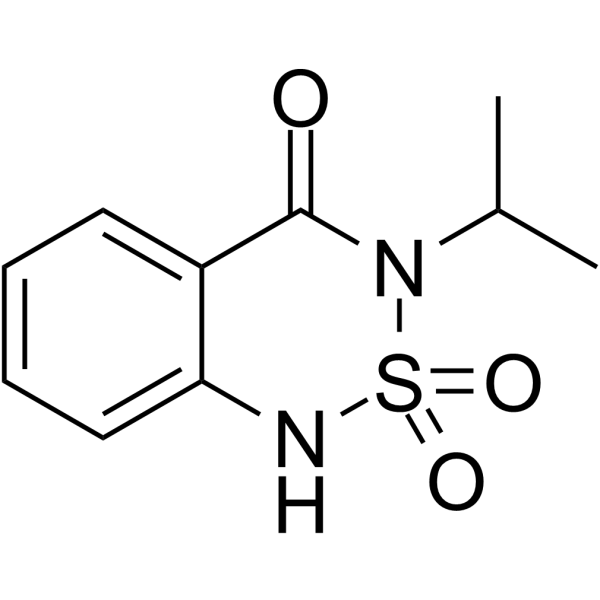
Bentazone is a benzothiadiazine that is 1H-2,1,3-benzothiadiazin-4(3H)-one 2,2-dioxide substituted by an isopropyl group at position 3. It has a role as an environmental contaminant, a xenobiotic and a herbicide.
Bentazon is a chemical manufactured by BASF Chemicals for use in herbicides. It is categorized under the thiadiazine group of chemicals. Sodium bentazon is available commercially and appears slightly brown in colour. Bentazon has been classified by the EPA as a Group E chemical, because it is believed to be non-carcinogenic to humans (as based on testing conducted on animals). However, there are no studies or experiments that can determine toxic and/or carcinogenic effects of bentazon on humans. Mechanism of Action Inhibition of photosynthesis at photosystem II. |
| 分子式 |
C10H12N2O3S
|
|---|---|
| 分子量 |
240.28
|
| 精确质量 |
240.056
|
| CAS号 |
25057-89-0
|
| 相关CAS号 |
Bentazone-13C10,15N;Bentazone-d7;131842-77-8
|
| PubChem CID |
2328
|
| 外观&性状 |
Colorless crystals; tech. is an ochre-yellow solid [
White, crystalline powder |
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
395.7±25.0 °C at 760 mmHg
|
| 熔点 |
137-139°C
|
| 闪点 |
193.1±23.2 °C
|
| 蒸汽压 |
0.0±0.9 mmHg at 25°C
|
| 折射率 |
1.583
|
| LogP |
2.8
|
| tPSA |
74.86
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
16
|
| 分子复杂度/Complexity |
385
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC(C)N1C(=O)C2=CC=CC=C2NS1(=O)=O
|
| InChi Key |
ZOMSMJKLGFBRBS-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C10H12N2O3S/c1-7(2)12-10(13)8-5-3-4-6-9(8)11-16(12,14)15/h3-7,11H,1-2H3
|
| 化学名 |
2,2-dioxo-3-propan-2-yl-1H-2λ6,1,3-benzothiadiazin-4-one
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.1618 mL | 20.8091 mL | 41.6181 mL | |
| 5 mM | 0.8324 mL | 4.1618 mL | 8.3236 mL | |
| 10 mM | 0.4162 mL | 2.0809 mL | 4.1618 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。