| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Bioavailability of oral dosing is 3% to 4% in horses. In humans at least 37% of oral benazepril is absorbed and reaches peak plasma concentration in 0.5 hours to 1 hour. Other studies have shown a peak plasma concentration at a median of 1.5 hours. Benazepril and benazeprilat are cleared predominantly by renal excretion in healthy subjects with normal renal function. Nonrenal (i.e., biliary) excretion accounts for approximately 11%-12% of benazeprilat excretion in healthy subjects. The final population pharmacokinetic model in one study estimated the volume of distribution to be 203±69.9L. The final population pharmacokinetic model of one study estimates the clearance to be 129±30.0L. /MILK/ Minimal amounts of unchanged benazepril and of benazeprilat are excreted into the breast milk of lactating women treated with benazepril. A newborn child ingesting entirely breast milk would receive less than 0.1% of the mg/kg maternal dose of benazepril and benazeprilat. Benazepril and benazeprilat are cleared predominantly by renal excretion. About 37% of an orally administered dose was recovered in urine as benazeprilat (20%), benazeprilat glucuronide (8%), benazepril glucuronide (4%) and as trace amounts of benazepril. Nonrenal (i.e., biliary) excretion accounts for approximately 11% - 12% of benazeprilat excretion. The effective half-life of benazeprilat following once daily repeat oral administration of benazepril hydrochloride is 10 to 11 hours. Thus, steady-state concentrations of benazeprilat should be reached after 2 or 3 doses of benazepril hydrochloride given once daily. Accumulation ratio based on AUC of benazeprilat was 1.19 following once daily administration. Metabolism / Metabolites Cleavage of the ester group (primarily in the liver) converts benazepril to its active metabolite, benazeprilat. Benazepril and benazeprilat are conjugated to glucuronic acid prior to urinary excretion. Benazepril and benazeprilat are cleared predominantly by renal excretion. About 37% of an orally administered dose was recovered in urine as benazeprilat (20%), benazeprilat glucuronide (8%), benazepril glucuronide (4%) and as trace amounts of benazepril. Nonrenal (i.e., biliary) excretion accounts for approximately 11% - 12% of benazeprilat excretion. The effective half-life of benazeprilat following once daily repeat oral administration of benazepril hydrochloride is 10 to 11 hours. Thus, steady-state concentrations of benazeprilat should be reached after 2 or 3 doses of benazepril hydrochloride given once daily. Accumulation ratio based on AUC of benazeprilat was 1.19 following once daily administration. After oral dosing in healthy dogs, benazepril is rapidly absorbed and converted into the active metabolite benazeprilat with peak levels of benazeprilat occurring approximately 75 minutes after dosing. Benazepril is almost completely metabolized to benazeprilat by cleavage of the ester group (primarily in liver). Both benazepril and benazeprilat undergo glucuronidation. Biological Half-Life The half life of the prodrug benazepril is 2.7±8.5h. The half life of the active metabolite benazeprilat is 22.3±9.2h The accumulation half life of benazepril is 10 to 11 hours. The elimination half life of benazeprilat is approximately 3.5 hours in healthy dogs. /Benazeprilat/ The effective half-life of benazeprilat following once daily repeat oral administration of benazepril hydrochloride is 10 to 11 hours. /Benazeprilat/ |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Benazepril is the nonsulfydryl angiotensin converting enzyme (ACE) inhibitor. It is used for treating hypertension and the management of heart failure in human patients and in animals. HUMAN STUDIES: Sensitivity reactions, including anaphylactoid reactions and angioedema (including laryngeal angioedema and tongue edema) are potentially fatal. Head and neck angioedema involving the tongue, glottis, or larynx may cause airway obstruction. Rare ACE inhibitor-associated clinical syndrome manifested initially by cholestatic jaundice may progress to fulminant hepatic necrosis and is potentially fatal. Patients receiving an ACE inhibitor, including benazepril, who develop jaundice or marked elevations of hepatic enzymes should discontinue the drug and receive appropriate monitoring. Human overdoses of benazepril have not been reported, but the most common manifestation of human benazepril overdosage is likely to be hypotension, for which the usual treatment would be intravenous infusion of normal saline solution. Hypotension can be associated with electrolyte disturbances and renal failure. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. ANIMAL STUDIES: Single oral doses of 3 g/kg benazepril were associated with significant lethality in mice. Rats, however, tolerated single oral doses of up to 6 g/kg. Reduced activity was seen at 1 g/kg in mice and at 5 g/kg in rats. In doses of 50-500 mg/kg/day benazepril had no adverse effect on the reproductive performance of male and female rats. No evidence of carcinogenicity was found when benazepril was administered to rats and mice for up to two years at doses of up to 150 mg/kg/day. No mutagenic activity was detected in the Ames test in bacteria (with or without metabolic activation), in an in vitro test for forward mutations in cultured mammalian cells, or in a nucleus anomaly test. Hepatotoxicity Benazepril, like other ACE inhibitors, has been associated with a low rate of serum aminotransferase elevations ( Likelihood score: D (possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because of the low levels of benazepril in breastmilk, amounts ingested by the infant are small and would not be expected to cause any adverse effects in breastfed infants. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Benazepril is 96.7% protein bound while benazeprilat is 95.3% protein bound. Interactions Because ACE inhibitors may promote kinin-mediated prostaglandin synthesis and/or release, concomitant administration of drugs that inhibit prostaglandin synthesis (e.g., aspirin, ibuprofen) may reduce the blood pressure response to ACE inhibitors, including enalapril. Limited data indicate that concomitant administration of ACE inhibitors with nonsteroidal anti-inflammatory agents (NSAIAs) occasionally may result in acute reduction of renal function; however, the possibility cannot be ruled out that one drug alone may cause such an effect. ... Aspirin and other NSAIAs also can attenuate the hemodynamic actions of ACE inhibitors in patients with congestive heart failure. Because ACE inhibitors share and enhance the effects of the compensatory hemodynamic mechanisms of heart failure, with aspirin and other NSAIAs interacting with the compensatory mechanisms rather than with a given ACE inhibitor per se, these desirable mechanisms are particularly susceptible to the interaction and a subsequent potential loss of clinical benefits. As a result, the more severe the heart failure and the more prominent the compensatory mechanisms, the more appreciable the interaction between NSAIAs and ACE inhibitors. Even if optimal dosage of an ACE inhibitor is used in the treatment of congestive heart failure, the potential cardiovascular and survival benefit may not be seen if the patient is receiving an NSAIA concomitantly. In several multicenter studies, concomitant admin of a NSAIA (i.e., a single 350-mg dose of aspirin) in patients with congestive heart failure inhibited favorable hemodynamic effects associated with ACE inhibitors, attenuating the favorable effects of these drugs on survival and cardiovascular morbidity. /ACE inhibitors/ Patients receiving coadministration of ACE inhibitor and mTOR inhibitor (e.g., temsirolimus, sirolimus, everolimus) therapy may be at increased risk for angioedema. Monitor for signs of angioedema. /ACE inhibitors/ Dual Blockade of the Renin-Angiotensin System (RAS) with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Most patients receiving the combination of two RAS inhibitors do not obtain any additional benefit compared to monotherapy. In general, avoid combined use of RAS inhibitors. Closely monitor blood pressure, renal function and electrolytes in patients on Lotensin and other agents that affect the RAS. Do not coadminister aliskiren with Lotensin in patients with diabetes. Avoid use of aliskiren with Lotensin in patients with renal impairment (GFR < 60 mL/min). Hypoglycemia has been reported rarely in diabetic patients receiving angiotensin-converting enzyme (ACE) inhibitors, including benazepril, concomitantly with insulin or oral antidiabetic agents. Patients receiving these drugs concomitantly should be informed of the possibility of hypoglycemia and monitored appropriately. For more Interactions (Complete) data for Benazepril (10 total), please visit the HSDB record page. |
| 参考文献 |
Arzneimittelforschung.1991 Jun;41(6):602-7;J Vet Med Sci.2007 Oct;69(10):1015-23.
|
| 其他信息 |
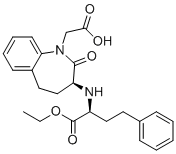
Benazepril is a benzazepine that is benazeprilat in which the carboxy group of the 2-amino-4-phenylbutanoic acid moiety has been converted to the corresponding ethyl ester. It is used (generally as its hydrochloride salt) as a prodrug for the angiotensin-converting enzyme inhibitor benazeprilat in the treatment of hypertension and heart failure. It has a role as an EC 3.4.15.1 (peptidyl-dipeptidase A) inhibitor and a prodrug. It is a benzazepine, a dicarboxylic acid monoester, an ethyl ester and a lactam. It is functionally related to a benazeprilat. It is a conjugate base of a benazepril(1+).
Benazepril, brand name Lotensin, is a medication used to treat high blood pressure (hypertension), congestive heart failure, and chronic renal failure. Upon cleavage of its ester group by the liver, benazepril is converted into its active form benazeprilat, a non-sulfhydryl angiotensin-converting enzyme (ACE) inhibitor. Benazepril is an Angiotensin Converting Enzyme Inhibitor. The mechanism of action of benazepril is as an Angiotensin-converting Enzyme Inhibitor. The physiologic effect of benazepril is by means of Decreased Blood Pressure. Benazepril is an angiotensin-converting enzyme (ACE) inhibitor widely used in the therapy of hypertension. Benazepril is associated with a low rate of transient serum aminotransferase elevations and has been linked to rare instances of acute liver injury. Benazepril is a carboxyl-containing angiotensin-converting enzyme (ACE) inhibitor with antihypertensive activity. As a prodrug, benazepril is metabolized to its active form benazeprilat. Benazeprilat competitively binds to and inhibits ACE, thereby blocking the conversion of angiotensin I to angiotensin II. This prevents the potent vasoconstrictive actions of angiotensin II resulting in vasodilation. Benazeprilat also decreases angiotensin II-induced aldosterone secretion by the adrenal cortex, which leads to an increase in sodium excretion and subsequently increases water outflow. See also: Benazepril Hydrochloride (has salt form); D&C Yellow No. 10 (active moiety of); Amlodipine besylate; benazepril hydrochloride (annotation moved to). Drug Indication Benazepril is indicated for the treatment of hypertension. It may be used alone or in combination with thiazide diuretics. FDA Label Mechanism of Action Benazeprilat, the active metabolite of Benazepril, competes with angiotensin I for binding at the angiotensin-converting enzyme, blocking the conversion of angiotensin I to angiotensin II. Inhibition of ACE results in decreased plasma angiotensin II. As angiotensin II is a vasoconstrictor and a negative-feedback mediator for renin activity, lower concentrations result in a decrease in blood pressure and stimulation of baroreceptor reflex mechanisms, which leads to decreased vasopressor activity and to decreased aldosterone secretion. Therapeutic Uses /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Benazepril is included in the database. Lotensin is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. ... It may be used alone or in combination with thiazide diuretics. /Included in US product label/ ACE inhibitors have been used in the management of heart failure, usually in conjunction with other agents such as cardiac glycosides, diuretics, and beta-blockers. /Angiotensin-converting enzyme (ACE) inhibitors; NOT included in US product label/ Both angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor antagonists have been shown to slow the rate of progression of renal disease in patients with diabetes mellitus and persistent albuminuria, and use of a drug from either class is recommended in such patients with modestly elevated (30-300 mg/24 hours) or higher (exceeding 300 mg/24 hours) levels of urinary albumin excretion. The usual precautions of ACE inhibitor or angiotensin II receptor antagonist therapy in patients with substantial renal impairment should be observed. /Angiotensin-converting enzyme (ACE) inhibitors; NOT included in US product label/ For more Therapeutic Uses (Complete) data for Benazepril (7 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ When pregnancy is detected, discontinue Lotensin as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. Rare angiotensin-converting enzyme (ACE) inhibitor-associated clinical syndrome manifested initially by cholestatic jaundice; may progress to fulminant hepatic necrosis and is potentially fatal. Patients receiving an ACE inhibitor, including benazepril, who develop jaundice or marked elevations of hepatic enzymes should discontinue the drug and receive appropriate monitoring. Serum potassium should be monitored periodically in patients receiving Lotensin. Drugs that inhibit the renin-angiotensin system can cause hyperkalemia. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of potassium-sparing diuretics, potassium supplements and/or potassium-containing salt substitutes. Adverse effects reported in greater than 1% of patients receiving benazepril include headache, dizziness, fatigue, somnolence, postural dizziness, nausea, and cough. Adverse effects reported in greater than 1% of patients receiving benazepril in fixed combination with hydrochlorothiazide include dizziness, fatigue, postural dizziness, headache, cough, hypertonia, vertigo, nausea, impotence, and somnolence. Adverse effects reported in greater than 1% of patients receiving benazepril in fixed combination with amlodipine include cough, headache, dizziness, and edema. For more Drug Warnings (Complete) data for Benazepril (16 total), please visit the HSDB record page. Pharmacodynamics Benazepril, an angiotensin-converting enzyme (ACE) inhibitor, is a prodrug which, when hydrolyzed by esterases to its active Benazeprilat, is used to treat hypertension and heart failure, to reduce proteinuria and renal disease in patients with nephropathies, and to prevent stroke, myocardial infarction, and cardiac death in high-risk patients. Benazepril and Benazeprilat inhibit angiotensin-converting enzyme (ACE) in human subjects and animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor substance, angiotensin II. Angiotensin II also stimulates aldosterone secretion by the adrenal cortex. |
| 分子式 |
C24H28N2O5
|
|---|---|
| 分子量 |
424.4895
|
| 精确质量 |
424.199
|
| CAS号 |
86541-75-5
|
| 相关CAS号 |
Benazepril hydrochloride;86541-74-4
|
| PubChem CID |
5362124
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
691.2±55.0 °C at 760 mmHg
|
| 熔点 |
133-135 °C(lit.)
|
| 闪点 |
371.8±31.5 °C
|
| 蒸汽压 |
0.0±2.3 mmHg at 25°C
|
| 折射率 |
1.608
|
| LogP |
3.86
|
| tPSA |
95.94
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
31
|
| 分子复杂度/Complexity |
619
|
| 定义原子立体中心数目 |
2
|
| SMILES |
C(N1C(=O)[C@@H](N[C@H](C(=O)OCC)CCC2C=CC=CC=2)CCC2C=CC=CC1=2)C(=O)O
|
| InChi Key |
XPCFTKFZXHTYIP-PMACEKPBSA-N
|
| InChi Code |
InChI=1S/C24H28N2O5/c1-2-31-24(30)20(14-12-17-8-4-3-5-9-17)25-19-15-13-18-10-6-7-11-21(18)26(23(19)29)16-22(27)28/h3-11,19-20,25H,2,12-16H2,1H3,(H,27,28)/t19-,20-/m0/s1
|
| 化学名 |
2-[(3S)-3-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]-2-oxo-4,5-dihydro-3H-1-benzazepin-1-yl]acetic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3558 mL | 11.7788 mL | 23.5577 mL | |
| 5 mM | 0.4712 mL | 2.3558 mL | 4.7115 mL | |
| 10 mM | 0.2356 mL | 1.1779 mL | 2.3558 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。