| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
ERα (IC50 = 26 nM); ERβ (IC50 = 99 nM)[1]
|
|---|---|
| 体外研究 (In Vitro) |
Bazedoxifeneacetate 是一种小分子 GP130 抑制剂,与 GP130 D1 结构域结合[1]。 Bazedoxifeneacetate 可抑制 GP130/STAT3 通路信号传导中由 Il-6 和 IL-11 产生的 STAT3 磷酸化 [1]。 Bazedoxifeneacetate(10μM -20μM;2小时)抑制人胰腺癌细胞中细胞因子诱导的STAT3磷酸化[2]。醋酸巴多昔芬(5-20 μM;过夜)会导致人胰腺癌细胞凋亡[2]。 Bazedoxifeneacetate 抑制 IL-6 引起的 STAT3 核转位 [2]。醋酸巴多昔芬通过抑制 GP130 来阻断胰腺癌细胞的细胞迁移[2]。
|
| 体内研究 (In Vivo) |
在未成熟大鼠子宫模型中, 巴多昔芬0.5和5.0 mg/kg)对子宫湿重的增加作用小于乙炔雌二醇(10微克/kg)或雷洛昔芬(0.5和5.0 mg/kg)。组织学分析显示,同时使用巴多昔芬也能减少雷洛昔芬刺激的子宫内膜上皮细胞和子宫肌层细胞肥大。在去卵巢大鼠中,与对照组相比,巴多昔芬与6周时骨矿物质密度显著增加有关,与去卵巢动物的样本相比,L4椎骨样本的抗压强度更好。在吗啡成瘾大鼠血管舒缩活性模型中,单独使用保骨剂量的巴多昔芬与17 - β -雌二醇对血管舒缩活性增加的抑制作用无关。醋酸巴多昔芬是一种很有前景的治疗骨质疏松症的新方法,与目前临床上使用的选择性雌激素受体调节剂相比,它对子宫和血管舒缩的影响可能更小。需要对照临床试验数据来证实这些效果。
巴泽多昔芬抑制小鼠体内模型capan-1肿瘤生长[2] 在小鼠模型中,醋酸巴多昔芬(5 mg/kg;搞笑;每日,持续18天)在体内抑制Capan-1肿瘤生长[2]。 在本研究中,研究人员验证了巴多昔芬是否在体内和体外抑制肿瘤生长。Capan-1细胞(3 × 106)注射方法如前面材料和方法所述。初始植入1周后,当肿瘤大小达到0.05 ~ 0.1cm3时,给予治疗组巴多昔芬5 mg/kg,对照组给予DMSO,连续18 d。如图6A所示,与载药组相比,巴多昔芬明显抑制肿瘤生长。巴多昔芬处理组肿瘤组织样本P-STAT3Y705降低,caspase-3被诱导(图6A),提示巴多昔芬可以抑制胰腺癌异种移植瘤的生长,诱导肿瘤细胞凋亡。 |
| 酶活实验 |
配体结合[1]
使用先前描述的[3H]-17β-雌二醇,采用固相竞争放射配体结合法评估醋酸巴多昔芬(BZA)与人ERα和ERβ的相互作用。 STAT3 DNA结合试验[2] 将BxPC-3细胞接种于10cm板上,用5-10 μmol/L的巴多昔芬或DMSO处理24h。细胞核提取试剂盒 按照制造商的方案制备细胞核提取液。采用STAT3 DNA结合ELISA试剂盒(Active Motif),采用ELISA方法分析核提取物的STAT3 DNA结合活性。在450nm处读取吸光度。 细胞因子或生长因子诱导STATs磷酸化[2] 将PANC-1、AsPC-1和hpf - ii胰腺癌细胞接种于10厘米板中,留置过夜。第二天晚上,这些细胞被血清饥饿。然后不处理细胞或用巴泽多西芬(5 ~ 20 μmol/L)或DMSO处理细胞。2小时后,未处理和巴多昔芬处理的细胞被IL6 (50 ng/mL)、IL11 (50 ng/mL)、OSM (50 ng/mL)或INFγ (50 ng/mL)刺激30分钟。收集细胞,用Western blot分析p-STAT3Y705或p-STAT1Y701。 |
| 细胞实验 |
蛋白质印迹分析[2]
细胞类型: AsPC-1 细胞 测试浓度: 10 μM、20 μM 孵育时间: 2 小时 实验结果: 抑制 IL-6、IL-11 或 OSM (50 ng/mL) 诱导的 STAT3 磷酸化。 细胞凋亡分析[2] 细胞类型: Capan-1 细胞、BxPC-3 细胞、HPAF-II 细胞、HPAC 细胞 测试浓度: 10μM、20μM(Capan-1); 5μM、10μM(BxPC-3); 10 μM、20 μM (HPAF-II); 10 μM、15 μM (HPAC) 孵育时间:过夜 实验结果:诱导细胞凋亡。 |
| 动物实验 |
Animal/Disease Models: 6weeks old female athymic nude mice[2]
Doses: 5 mg /kg Route of Administration: po (oral gavage), daily, for 18 days Experimental Results: Suppressed pancreatic cancer xenograft tumor growth and induced apoptosis in tumor cells. Vasomotor instability (hot flush)[1] Ovariectomized female (60 d) rats were obtained after surgery. The surgeries were performed minimally 7 d before initiation of any experiment. Vehicle and ethinyl estradiol (0.3 mg/kg) were included in each replicate. Bazedoxifene was administered orally in a saline, Tween-80, methylcellulose vehicle. A detailed description of methodology for evaluating vasomotor instability in rats has been published (21). Briefly, compound treatment (17β-estradiol, ethinyl estradiol, or bazedoxifene) is initiated, and on the third day of treatment each animal receives a morphine pellet sc. This is followed by two more pellets on the fifth day of treatment. On the eighth day, a thermistor is taped to the animal’s tail to measure tail skin temperature for 15 min (to obtain baseline temperature) followed by a sc injection of naloxone (1 mg/kg). Tail skin temperature readings continue for 1 h after naloxone injection. All animal studies were conducted in accordance with the principles and standard procedures approved by IACUC of the Research Institute at Nationwide Children's Hospital. Capan-1 (3 × 106) and HPAF-II (3 × 106) cells in Matrigel were injected subcutaneously into the both side of flank area of 6-week-old female athymic nude mice which were purchased from Harlan. After Capan-1 tumor development, which was 1 week after initial implantation, mice were divided into two treatment groups consisting of four mice (tumors: n = 8): DMSO vehicle control and gavage injection of Bazedoxifene (5 mg/kg/d). Mice bearing HPAF-II tumor were irrigated with Bazedoxifene(5 mg/kg/d) and/or injected via abdomen with paclitaxel (15 mg/kg, 2/w). Tumor growth was determined by measured the length (L) and width (W) of the tumor every other day with a caliper, and tumor volume was calculated on the basis of the following formula: volume = 0.52 × LW2. After 21 days of treatment, tumors were harvested, snap-frozen in dry ice, and stored at −80°C. Tumors tissue homogenates were lysed and separated by SDS-PAGE to examine the expression of STAT3 phosphorylation, P-ERK1/2, P-AKT (Ser473), and cleaved caspase-3.[2] |
| 参考文献 | |
| 其他信息 |
See also: Bazedoxifene (annotation moved to).
Drug Indication Duavive is indicated for: Treatment of oestrogen deficiency symptoms in postmenopausal women with a uterus (with at least 12 months since the last menses) for whom treatment with progestin-containing therapy is not appropriate. The experience treating women older than 65 years is limited. Conbriza is indicated for the treatment of postmenopausal osteoporosis in women at increased risk of fracture. A significant reduction in the incidence of vertebral fractures has been demonstrated; efficacy on hip fractures has not been established. When determining the choice of Conbriza or other therapies, including oestrogens, for an individual postmenopausal woman, consideration should be given to menopausal symptoms, effects on uterine and breast tissues, and cardiovascular risks and benefits. |
| 分子式 |
C30H34N2O3.HCL
|
|
|---|---|---|
| 分子量 |
507.06
|
|
| 精确质量 |
530.278
|
|
| 元素分析 |
C, 72.43; H, 7.22; N, 5.28; O, 15.07
|
|
| CAS号 |
198481-33-3
|
|
| 相关CAS号 |
Bazedoxifene;198481-32-2;Bazedoxifene hydrochloride;198480-56-7
|
|
| PubChem CID |
154256
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 沸点 |
694.4ºC at 760 mmHg
|
|
| 闪点 |
373.8ºC
|
|
| 蒸汽压 |
6.33E-20mmHg at 25°C
|
|
| LogP |
6.359
|
|
| tPSA |
95.16
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
7
|
|
| 重原子数目 |
39
|
|
| 分子复杂度/Complexity |
654
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
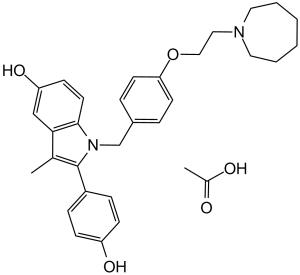
CC1=C(C2=CC=C(O)C=C2)N(CC3=CC=C(OCCN4CCCCCC4)C=C3)C5=CC=C(O)C=C51.CC(O)=O
|
|
| InChi Key |
OMZAMQFQZMUNTP-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C30H34N2O3.C2H4O2/c1-22-28-20-26(34)12-15-29(28)32(30(22)24-8-10-25(33)11-9-24)21-23-6-13-27(14-7-23)35-19-18-31-16-4-2-3-5-17-31;1-2(3)4/h6-15,20,33-34H,2-5,16-19,21H2,1H3;1H3,(H,3,4)
|
|
| 化学名 |
1-(p-(2-(Hexahydro-1H-azepin-1-yl)ethoxy)benzyl)-2-(p-hydroxyphenyl)-3-methylindol-5-ol acetic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.75 mg/mL (5.18 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.75 mg/mL (5.18 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.71 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.5 mg/mL (4.71 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100μL 25.0mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: ≥ 2.5 mg/mL (4.71 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9722 mL | 9.8608 mL | 19.7215 mL | |
| 5 mM | 0.3944 mL | 1.9722 mL | 3.9443 mL | |
| 10 mM | 0.1972 mL | 0.9861 mL | 1.9722 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。