| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
ATR (IC50 = 7 nM)
|
|---|---|
| 体外研究 (In Vitro) |
Elimusertib 有效抑制广谱人类肿瘤细胞系的增殖,中值 IC50 为 78 nM[1]。 Elimusertib 对羟基脲诱导的 H2AX 磷酸化具有有效的抑制作用 (IC50: 36 nM)[1]。 Elimusertib 对 mTOR 显示出良好的选择性(IC50 值比:mTOR/ATR 61)[3]。 Elimusertib 对其他相关激酶表现出高选择性,包括 DNA-PK、ATM 和 PI3K(IC50 值分别为 332 nM、1420 nM 和 3270 nM)[3]。 Elimusertib 在体外对多种癌细胞系表现出强大的抗增殖活性,包括 CRC 细胞系 HT-29 和 LoVo 以及 B 细胞淋巴瘤细胞系 SU-DHL-8 (IC50: 9 nM)[3]。
|
| 体内研究 (In Vivo) |
Elimusertib 可导致套细胞淋巴瘤模型中的肿瘤完全缓解,并在多种卵巢癌和结直肠癌异种移植模型中的单一疗法中表现出有效的抗肿瘤功效[2]。 Elimusertib(50 mg/kg;PO;bid;用药 3 天/停药 4 天;持续 11 天)在 ATM 突变的 SU-DHL-8 (ATM K1964E) 人 GCB-DLBCL 细胞系衍生的异种移植模型中表现出有效的抗肿瘤活性在小鼠中[3]。 NOD/SCID 小鼠中铂抗性 ATM 蛋白低表达 CR5038 人 CRC PDX 模型与 Elimusertib(20 mg/kg,第 14 天起 10 mg/kg;口服;每天;2 天/5)联合显示出协同抗肿瘤活性。休息日;42 天)[3]。 Elimusertib 口服给药(大鼠和狗 0.6-1 mg/kg)后表现出中等的口服生物利用度(大鼠 87%,狗 51%)[3]。 Elimusertib 在静脉内给药(小鼠、大鼠、狗 0.3-0.5 mg/kg)[3]。
|
| 酶活实验 |
BAY 1895344的亲和力和选择性[2]
基于时间分辨荧光共振能量转移(TR-FRET)的ATR竞争结合分析用于使用荧光5-TAMRA标记的示踪剂1(一种ATP竞争性ATRi)确定BAY 1895344与ATR的亲和力。570和545 nm的发射比用于评估BAY 1895344与ATR的结合亲和力。 如前所述,使用内部激酶小组和由468种激酶组成的激酶扫描分析小组(DiscoverX)评估BAY 1895344的选择性。 通过分别测量羟基脲处理的HT-29细胞和新卡司他丁处理的M059J细胞中磷酸化Ser139组蛋白H2AX(γH2AX)水平来确定ATR和ATM激酶的活性。通过测量AKT磷酸化,研究MCF7乳腺癌症细胞中PI3K/AKT/mTOR信号通路的活性。 |
| 细胞实验 |
BAY 1895344 的抗增殖活性针对一组 38 种癌细胞系进行了评估。 BAY1895344 暴露 72 至 96 小时后,评估细胞增殖。 CellTiter-Glo 细胞活力测定或结晶紫染色用于测量细胞活力。
BAY 1895344的抗增殖活性针对38种癌症细胞系进行评估(补充表S3)。在暴露于BAY 1895344 72至96小时后测量细胞增殖。使用结晶紫染色或CellTiter Glo细胞活力测定法测定细胞活力。[2] 通过测定联合指数(CI)来评估BAY 1895344与不同药物联合使用的抗增殖活性。在HT-29细胞中研究了BAY 1895344(3-300 nmol/L)与顺铂(100 nmol/L-10 nmol/L。在合成雄激素甲基三烯酮R1881(10 nmol/L)存在的情况下,在LAPC-4细胞中进行了BAY 1895344(10 nmol/L–10μmol/L)和达罗鲁胺(10 nmool/L–10μmol/L)的联合研究。在一组癌症细胞系中对BAY 1895344和一些化合物进行了额外的组合研究(补充表S4)。用单一化合物或固定化合物比例的组合处理细胞4至6天,并使用CellTiter Glo测量存活率。EC50值由每个单独组合数据点的三份值计算得出,并生成了相应的等压线图。根据中位效应模型计算CI(33)。CI≤0.8被定义为大于加性(即协同)相互作用,CI≥1.2被定义为拮抗相互作用。[2] 克隆形成联合试验用于评估BAY 1895344的放射增敏潜力。用3 nmol/L BAY 1895344和不同强度的γ辐射处理LOVO结直肠癌癌症细胞,使其形成集落10至14天,最后计数集落以计算联合效应。[2] |
| 动物实验 |
female SCID beige mice, female C.B-17 SCID mice, male NMRI nude mice, female NMRI nude mice
50 mg/kg Oral gavage In vivo studies in CDX models[2] All animal experiments were conducted in accordance with the German Animal Welfare Act and approved by local authorities. The in vivo antitumor efficacy and tolerability of BAY 1895344 as monotherapy/combination therapy were evaluated in CDX subcutaneous or orthotopic xenograft models in mice. Monotherapy experiments were performed in GRANTA-519 (in female SCID beige mice), REC-1 (in female C.B-17 SCID mice), PC-3 (in male NMRI nude mice), LOVO, and A2780 (both in female NMRI nude mice) models treated with BAY 1895344 at 50 mg/kg [all models; twice daily, 3 days on/4 days off (3on/4off), per os/orally] or at 3, 10, or 30 mg/kg (GRANTA-519; twice daily, 3on/4off, per os/orally), ibrutinib (REC-1; 20 mg/kg, once daily, per os/orally), AZD6738 (GRANTA-519, REC-1; 50 mg/kg, once daily, per os/orally), M6620 (GRANTA-519 and REC-1; 100 mg/kg, once daily, per os/orally), or 5-FU (LOVO; 50 mg/kg, once weekly, intraperitoneally). The combination of BAY 1895344 at 10 or 20 mg/kg [once daily, 2 days on/5 days off (2on/5off), per os/orally.] or 50 mg/kg (twice daily, 3on/4off, per os/orally) and carboplatin (50 mg/kg, once weekly, intraperitoneally) was investigated in IGROV-1 tumor–bearing female nude (nu/nu) mice. The combination of 20 or 50 mg/kg BAY 1895344 (twice daily, 2on/5off, per os/orally) and EBRT (5 Gy, 7.7 minutes, once daily on days 12 and 27) was investigated in LOVO tumor–bearing female NMRI nude mice. Combination therapy experiments with 20 or 50 mg/kg BAY 1895344 (twice daily, 3on/4off, per os/orally) and 20 or 50 mg/kg olaparib (once daily, intraperitoneally) were performed in MDA-MB-436 and 22Rv1 models in female NOD/SCID and male SCID mice, respectively. Combination experiments with 20 mg/kg BAY 1895344 (twice daily, 3on/4off, per os/orally) and 100 mg/kg darolutamide (once daily, per os/orally) were performed in the hormone-dependent LAPC-4 prostate cancer model in male C.B-17 SCID mice. Castrated mice served here as a control. For a triple combination treatment, mice received EBRT (5 Gy, every 7 days twice) in addition to treatment with BAY 1895344 and darolutamide. To elucidate the in vivo mode of action of BAY 1895344, ATR and H2AX phosphorylation was determined in lysed GRANTA-519 xenograft tumor samples. For the quantification of circulating ATRis, plasma samples were taken from mice and measured by LC-MS/MS. |
| 参考文献 | |
| 其他信息 |
Elimusertib is an orally available ataxia telangiectasia and Rad3-related (ATR)-specific kinase inhibitor, with potential antineoplastic activity. Upon oral administration, elimusertib selectively binds to and inhibits the activity of ATR, which prevents ATR-mediated signaling. This inhibits DNA damage checkpoint activation, disrupts DNA damage repair and induces apoptosis in ATR-overexpressing tumor cells. ATR, a serine/threonine protein kinase upregulated in a variety of cancer cell types, plays a key role in DNA repair, cell cycle progression and cell survival.
The integrity of the genome of eukaryotic cells is secured by complex signaling pathways, known as DNA damage response (DDR). Recognition of DNA damage activates DDR pathways resulting in cell cycle arrest, suppression of general translation, induction of DNA repair, cell survival or even cell death. Proteins that directly recognize aberrant DNA structures recruit and activate kinases of the DDR pathway, such as ATR (ataxia telangiectasia and Rad3-related). ATR responds to a broad spectrum of DNA damage, including double-strand breaks (DSB) and lesions derived from interference with DNA replication as well as increased replication stress (e.g. in oncogene-driven tumor cells). Therefore, inhibition of ATR kinase activity could be the basis for a novel anti-cancer therapy in tumors with increased DNA damage, deficiency in DNA damage repair or replication stress. Herein we report the identification of the potent, highly selective and orally available ATR inhibitor BAY 1895344 by a collaborative effort involving medicinal chemistry, pharmacology, DMPK and computational chemistry. The chemical structures of lead compound BAY-937 and clinical candidate BAY 1895344 as well as the main SAR trends within this novel class of naphthyridine derivatives will be disclosed for the first time. The novel lead compound BAY-937 revealed promising inhibition of ATR (IC50 = 78 nM) and high kinase selectivity in vitro. In cellular mechanistic assays BAY-937 inhibited hydroxyurea-induced H2AX phosphorylation (IC50 = 380 nM) demonstrating the anticipated mode of action. Moreover, BAY-937 was shown to inhibit proliferation of a variety of tumor cell lines with low- to sub-micromolar IC50 values. In initial xenograft studies, BAY-937 revealed moderate activity in monotherapy and in combination with cis-platin. However, BAY-937 also revealed low aqueous solubility, low bioavailability (rat) and activity in the hERG patch clamp assay. Extensive lead optimization efforts led to the identification of the novel, orally available ATR inhibitor BAY 1895344. In vitro, BAY 1895344 was shown to be a very potent and highly selective ATR inhibitor (IC50 = 7 nM), which potently inhibits proliferation of a broad spectrum of human tumor cell lines (median IC50 = 78 nM). In cellular mechanistic assays BAY 1895344 potently inhibited hydroxyurea-induced H2AX phosphorylation (IC50 = 36 nM). Moreover, BAY 1895344 revealed significantly improved aqueous solubility, bioavailability across species and no activity in the hERG patch-clamp assay. BAY 1895344 also demonstrated very promising efficacy in monotherapy in DNA damage deficient tumor models as well as combination treatment with DNA damage inducing therapies. The start of clinical investigation of BAY 1895344 is planned for early 2017. [1] The DNA damage response (DDR) secures the integrity of the genome of eukaryotic cells. DDR deficiencies can promote tumorigenesis but concurrently may increase dependence on alternative repair pathways. The ataxia telangiectasia and Rad3-related (ATR) kinase plays a central role in the DDR by activating essential signaling pathways of DNA damage repair. Here, we studied the effect of the novel selective ATR kinase inhibitor BAY 1895344 on tumor cell growth and viability. Potent antiproliferative activity was demonstrated in a broad spectrum of human tumor cell lines. BAY 1895344 exhibited strong monotherapy efficacy in cancer xenograft models that carry DNA damage repair deficiencies. The combination of BAY 1895344 with DNA damage-inducing chemotherapy or external beam radiotherapy (EBRT) showed synergistic antitumor activity. Combination treatment with BAY 1895344 and DDR inhibitors achieved strong synergistic antiproliferative activity in vitro, and combined inhibition of ATR and PARP signaling using olaparib demonstrated synergistic antitumor activity in vivo Furthermore, the combination of BAY 1895344 with the novel, nonsteroidal androgen receptor antagonist darolutamide resulted in significantly improved antitumor efficacy compared with respective single-agent treatments in hormone-dependent prostate cancer, and addition of EBRT resulted in even further enhanced antitumor efficacy. Thus, the ATR inhibitor BAY 1895344 may provide new therapeutic options for the treatment of cancers with certain DDR deficiencies in monotherapy and in combination with DNA damage-inducing or DNA repair-compromising cancer therapies by improving their efficacy.[2] |
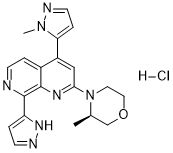
| 分子式 |
C20H21N7O
|
|---|---|
| 分子量 |
375.43
|
| 精确质量 |
411.15743
|
| 元素分析 |
C, 58.32; H, 5.38; Cl, 8.61; N, 23.80; O, 3.88
|
| CAS号 |
1876467-74-1
|
| 相关CAS号 |
Elimusertib hydrochloride;Elimusertib-d3
|
| PubChem CID |
118869362
|
| 外观&性状 |
Yellow solid powder
|
| tPSA |
84.8Ų
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
537
|
| 定义原子立体中心数目 |
1
|
| InChi Key |
YBXRSCXGRPSTMW-CYBMUJFWSA-N
|
| InChi Code |
InChI=1S/C20H21N7O/c1-13-12-28-10-9-27(13)18-11-15(17-5-8-23-26(17)2)14-3-6-21-20(19(14)24-18)16-4-7-22-25-16/h3-8,11,13H,9-10,12H2,1-2H3,(H,22,25)/t13-/m1/s1
|
| 化学名 |
(3R)-3-methyl-4-[4-(2-methylpyrazol-3-yl)-8-(1H-pyrazol-5-yl)-1,7-naphthyridin-2-yl]morpholine
|
| 别名 |
Elimusertib; HCl; BAY-1895344 HCl; BAY1895344 HCl; BAY 1895344 HCl; 7N13IK9LNH; BAY1895344; (R)-3-methyl-4-(4-(1-methyl-1H-pyrazol-5-yl)-8-(1H-pyrazol-3-yl)-1,7-naphthyridin-2-yl)morpholine;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~5.4 mg/mL (~14.38 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 1.09 mg/mL (2.90 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 10.9mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: 0.89 mg/mL (2.37 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 8.9 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: 4 mg/mL (10.65 mM) in 0.5% CMC-Na/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6636 mL | 13.3181 mL | 26.6361 mL | |
| 5 mM | 0.5327 mL | 2.6636 mL | 5.3272 mL | |
| 10 mM | 0.2664 mL | 1.3318 mL | 2.6636 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
TIC10 induces TRAIL in tumor and normal cells. |
TIC10 is effective as an antitumor agent in GBM. |