| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
NF-κB
|
|---|---|
| 体外研究 (In Vitro) |
Barlerin(8-O-乙酰山枝苷甲酯)处理可抑制 SH-SY5Y 细胞中 TNF 诱导的核转录因子 B (NF-κB) 激活并减少高迁移率组 box-1 (HMGB-1) 表达。 [1]。通过阻止高迁移率族蛋白 1 (HMGB1) 的表达,用 barlerin(8-O-乙酰山芝苷甲酯)9 μM 处理 H9c2 细胞可阻止 TNF-α 诱导的 NF-κB 磷酸化[2]。
8- o -乙酰山芝苷甲酯(ND01)对NF-κB活化和HMGB-1表达的影响[1] NF-κB通路在细胞因子的分泌中起关键作用。在细胞核中测定p50和p65的数量。TNF-α刺激导致NF-κB转录因子p50/p65的强烈激活。然而,这种激活被ND01部分阻断,如图1所示。[1] 同时,还研究了作为NF-κB另一活化因子的i -κB激酶系统。TNF-α刺激导致i - κ b明显降解。这种降解被ND01抑制(图2)。此外,p- i - κ b的磷酸化被TNF-α增加,也被ND01抑制。[1] 在非tnf -α刺激的SH-SY5Y细胞中,磷酸化nf -κB表达较低。然而,TNF-α 20 ng/ml刺激SH-SY5Y细胞120 min后,磷酸化- nf -κB表达显著增加。我们比较了ND01对TNF-α诱导的SH-SY5Y细胞中磷酸化- nf -κB和HMGB-1表达水平的影响,以及选择性HMGB-1抑制剂甘草酸的影响。图2结果显示,甘草酸(100 μM)预处理SH-SY5Y细胞120 min,可阻断TNF-α-诱导的HMGB-1表达,降低NF-κB磷酸化。用ND01 10 μM预处理SH-SY5Y细胞,可阻断TNF-α-诱导的NF-κB磷酸化,降低HMGB-1表达,如图2所示。[1] |
| 体内研究 (In Vivo) |
Barlerin(8-O-乙酰山栀苷甲酯)40 mg/kg 即使在 I/R 后 4 小时给药也表现出显着的神经保护作用。在缺血性脑组织中,barlerin 40 mg/kg 可抑制 NF-κB 激活,降低 HMGB-1 表达,减轻组织病理学损伤,并减轻脑肿胀[1]。 barlerin(8-O-乙酰山栀苷甲酯)可显着加速缺血脑中的血管生成,这也能促进中风后的功能恢复。此外,与载体治疗相比,barlerin 显着促进血管化。它提高 Ang1、Tie2 和 Akt VEGF 的水平及其表达[3]。小鼠体内活化的部分凝血活酶、凝血酶原和凝血酶时间不受barlerin(8-O-乙酰山栀苷甲酯)的影响,但毛细血管凝血时间和失血量显着缩短。在纤溶亢进小鼠中,它显着延长了优球蛋白凝块溶解所需的时间[4]。
炎症激活在卒中和糖尿病(DM)的病理生理机制中起着至关重要的作用,对脑的进展产生有害影响,导致糖尿病卒中血管损伤。本研究旨在探讨8- o -乙酰山枝八苷甲酯(ND01)对肿瘤坏死因子-α (TNF-α)刺激的SH-SY5Y细胞株和体内实验性缺血性糖尿病脑卒中模型的影响。TNF-α刺激的SH-SY5Y细胞用ND01预孵育,然后分析蛋白表达。体内实验:糖尿病大鼠大脑中动脉闭塞(MCAO) 30 min,再灌注23 h。ND01处理SH-SY5Y细胞阻断TNF-α-诱导的核转录因子κB (NF-κB)活化,降低高迁移率组盒-1 (HMGB-1)表达。ND01 40 mg/kg即使在I/R后延迟给药4小时也具有显著的神经保护作用。ND01 40 mg/kg可减轻缺血脑组织组织病理损伤,减轻脑肿胀,抑制NF-κB活化,降低HMGB-1表达。这些数据表明ND01通过减轻糖尿病脑I/R损伤和减弱血脑屏障(BBB)破坏,具有良好的治疗时间窗,其保护作用可能涉及HMGB-1和NF-κB信号通路。[1] 8-O-acetyl shanzhiside methylester (ND01)/8- o -乙酰山芝苷甲基酯(ND01)是从圆叶榄(Lamiophlomis rotata, Benth.)中分离得到的。奖赏。本研究探讨ND01在体内抗心肌缺血再灌注(I/R)损伤的作用,并阐明其在体外的可能机制。结果表明,ND01对H9c2细胞缺氧诱导的细胞毒性具有浓度依赖性。ND01 9 μM处理H9c2细胞,通过阻断高迁移率组框1 (High-mobility group box1, HMGB1)表达,阻断TNF-α-诱导的核因子κ b (NF-κB)磷酸化。ND01 10mg/kg(静脉注射)对大鼠心肌I/R损伤有保护作用,梗死面积减少,血流动力学改善,心肌损伤严重程度减轻。ND01还能降低血清促炎因子水平,降低缺血心肌组织中高迁移率组蛋白(HMGB1)和磷酸化NF-κB的表达。此外,连续静脉注射ND01 14天可减轻心脏重构。这些保护作用表明ND01可能是通过hmgb1依赖性NF-κB信号通路阻断心肌炎症级联反应。[2] 研究人员研究了8- o -乙酰山芝苷甲基酯(ND01)是否调节血管生成,从而改善脑卒中后的功能结局。成年雄性大鼠大脑中动脉闭塞(MCAO)和再灌注1小时后,在缺血再灌注(I/R)后24小时开始,每天静脉注射不同剂量(5和10 mg/kg)的ND01,连续14天。行神经功能检查,测定脑Evans蓝外渗。分别用免疫组织化学和Western blot检测血管生成和血管生成因子的表达。结果表明,ND01可显著促进缺血脑的血管生成,改善脑卒中后的功能结局。与载药处理相比,ND01也显著增加了血管化。ND01增加VEGF、Ang1的表达、Tie2和Akt VEGF的磷酸化。Ang1/Tie2轴和Akt通路介导nd01诱导的血管生成。[3] 抗纤溶活性来源于8- o -乙酰山芝苷甲酯(ASM),它是龙葵中含量最高的环烯醚萜类苷之一。ASM可显著缩短CBCT (P<0.05),减少体内失血量,但对小鼠APTT、PT、TT无影响。特别是,它显著延长了高纤溶小鼠的ECLT。表明ASM具有抑制纤溶的作用。ASM对高纤溶小鼠CBCT、创伤性出血量和ECLT均有影响。 结论:ASM是轮状螺旋体的主要止血化合物。ASM的止血机制是通过抗纤溶活性实现的。ASM是一种新型的环烯醚萜苷类纤维蛋白溶解抑制剂。[4] |
| 酶活实验 |
NF-κ b结合试验[1]
SH-SY5Y细胞(5 × 106)用8-O-acetyl shanzhiside methylester (ND01) 10 μM预孵育22小时,然后用TNF-α (20 ng/ml)孵育1或2小时,PBS洗涤1次,将细胞刮入1 ml冷PBS中离心成球。核提取物的制备方法如上所述12。采用ELISA试剂盒检测NF-κB (p50/p65)的dna结合活性。 <人力资源> 8-O-acetyl shanzhiside methylester (ND01)抑制NF-κB活化和HMGB1表达[2] 我们比较了ND01对HMGB1选择性抑制剂甘草酸(100 μM)和NF-κB对TNF-α-诱导的H9c2细胞NF-κB和HMGB1 (20 ng/ml, 30 min)活化的影响。 |
| 细胞实验 |
在缺氧前,用 Barlerin(8-O-乙酰山栀苷甲酯)以一定浓度(1、3、9 和 27 27μM)预处理细胞 24 小时。 MTT 测定用于评估细胞活力[2]。
TNF-α-刺激SH-SY5Y细胞NF-κB活化及HMGB-1表达[1] 人神经母细胞瘤(SH-SY5Y)细胞培养于F12+ DMEM (1:1, v/v)培养基中,添加10%磷酸缓冲盐水(PBS)和1%青霉素/链霉素。细胞保存在5% CO2/95% O2的加湿培养箱中,温度37°C。分离的细胞以5 × 105/cm2的密度接种于聚赖氨酸包被板上,在DMEM中培养,添加10% (v/v)马血清、5% (v/v)胎牛血清、100 U/ml青霉素和0.1 mg/ml链霉素。新鲜培养基每周更换两次。[1] 体外TNF-α刺激SH-SY5Y细胞系实验,将SH-SY5Y细胞(5 × 106)与8-O-acetyl shanzhiside methylester (ND01)(9 μM)或HMGB-1抑制剂甘草酸苷(100 μM)预孵育120 min,然后与TNF-α (20 ng/ml)孵育30 min, CO2培养箱培养12 h。细胞用冰上的冷冻PBS洗涤2次,在NP40裂解缓冲液中裂解,缓冲液为50 mM Tris, pH 7.4, 250 mM NaCl, 5 mM EDTA, 50 mM NaF, 1 mM Na3VO4, 1% NP-40和0.02% NaN3,并添加1 mM PMSF和1 ×蛋白酶抑制剂鸡尾酒。等量的细胞蛋白(50 μg)通过SDS-PAGE分离,用特异性抗体HMGB-1、i -κB、磷酸化- i -κB -α、磷酸化- nf -κB和增殖细胞核抗原(PCNA,加载对照)进行Western blot分析。用Gel Doc 2000扫描并定量了条带的光密度。根据相应的PCNA波段数据进行归一化处理。结果表示为比对照增加了两倍。 <人力资源> 8-O-acetyl shanzhiside methylester (ND01)减弱缺氧诱导的细胞毒性[2] 细胞活力测定结果如图1所示。缺氧6h后,与对照细胞相比,存活率仅为52.0±8.9%。8-O-acetyl shanzhiside methylester (ND01)(3、9和27 μM)对缺氧诱导的细胞损伤呈浓度依赖性,使细胞存活率分别恢复到63.7±6.0%、69.9±8.6%和74.4±9.6%。 |
| 动物实验 |
Rats: In saline, Barlerin (8-O-Acetyl shanzhiside methyl ester) is made. Adult male rats are given an hour of middle cerebral artery occlusion (MCAO) and reperfusion. They are then given different doses of 8-O-acetyl shanzhiside methylester (ND01) (5 and 10 mg/kg) intravenously every day for 14 days, starting 24 hours after the I/R (ischaemia and reperfusion). In addition to measuring cerebral Evans blue extravasation, neurological functional tests are carried out[3].
Mouse: In saline, Barlerin (8-O-Acetyl shanzhiside methyl ester) is made. The five groups (saline group, Hemocoagulase, 0.34 KU/kg, intravenous, ASM-L, 100 mg/kg, ASM-M, 250 mg/kg, and ASM-H, 500 mg/kg) of male Balb/C mice (20 to 25g) are assigned at random. Five minutes before sodium pentobarbital (200 mg/kg, i.p.) is used to anesthetize the patient, the drugs and the vehicle are injected through the vena cauda. After the injection, blood is drawn from the heart 20 minutes later. Prothrombin time, thrombin time, fibrinogen, and activated partial thromboplastin time are measured[4]. Streptozotocin (STZ)-induced diabetes model and rat cerebral ischaemia study protocol [1] Two hundred rats (fasting for 20 hr) were induced by single i.p. STZ at a dose of 50 mg/kg. STZ was diluted in citrate buffer 0.1 M (pH 4.0). After STZ injection for 3 weeks, rats with glycaemia value between 12.0 and 20.0 mM were used. The diabetic rats were anaesthetized with chloral hydrate (350 mg/kg, i.p.). Rectal temperature was recorded and maintained at 37°C throughout the surgical procedure. The operation of MCAO was carried out according to previous procedures with minor modifications 13. The left common carotid artery was occluded, and the branches of the external carotid artery were dissected and divided. The internal carotid artery was followed rostrally, and a 4-0 filament (the diameter of the filament was 0.25, but the diameter of the tip was 0.34 mm to create a globular stopper) was introduced into the internal carotid artery and advanced until resistance was felt. The filament was removed after 30 min. The rats were kept under conditions of controlled temperature (24–25°C) for the first 23 hr after surgery. [1] A pilot study was conducted with four different doses of 8-O-acetyl shanzhiside methylester (ND01) (10, 20, 40 or 80 mg/kg) to determine the dose-dependent effect in the acute I/R-treated diabetic rats. It was observed that 8-O-acetyl shanzhiside methylester (ND01) at doses of 20, 40 and 80 mg/kg significantly (p < 0.05) lowered infarct volume and neurological deficit scores of the acute I/R-induced diabetic rats after 23 hr of the experiment. Hence, 8-O-acetyl shanzhiside methylester (ND01) 40 mg/kg was chosen for this study. [1] For therapeutic time-window studies, 40 MCAO diabetic rats were randomly divided into four groups of 10 rats each plus 10 diabetic rats as control. Rats received dose of 40 mg/kg by intravenous bolus injection into the tail vein 2, 4 and 6 hr after reperfusion. Vehicle-treated rats were administered with saline. Neurological deficits were determined 23 hr after ischaemia followed by brain infarct examination. [1] For anti-inflammatory mechanism studies, 54 rats were randomly divided into three subgroups of 18 rats each plus 18 rats as control (non-diabetic). Rats received a dose of 40 mg/kg intravenous bolus injection into the tail vein 30 min. after reperfusion. Diabetic or vehicle-treated rats were administered with saline. All the brain were evaluated by Evans blue extravasation, then analysed HMGB1, IκB, phosphor-IκB-α, phosphor-NF-κB by Western blot and the histopathological damage were evaluated by NeuN staining, especially. [1] For long-term studies, 20 rats were randomly divided into two groups of 10 rats each. Rats received a dose of 40 mg/kg by intravenous bolus injection into the tail vein 30 min. after reperfusion. The vehicle-treated rats were administered with saline. Neurological deficits were determined on days 3, 7 and 14 after I/R. Fourteen days after I/R, eight rats were stained in the 8-O-acetyl shanzhiside methylester (ND01) 40 mg/kg group, and six rats were stained in the vehicle-treated group. The brain infarct was examined according to a previous method. [1] I/R procedure to induce cerebral ischaemia. [3] The body weight of rats was 280–320 g. After 1 week of acclimatization, rats were anaesthetized with chloral hydrate (350 mg/kg, i.p.). The middle cerebral artery occlusion (MCAO) operation was performed according to procedures described previously. The left common carotid artery was occluded, and the branches of the external carotid artery were dissected and divided. The internal carotid artery was followed rostrally and a 4–0 filament (the diameter of the filament is 0.25, but the diameter of the tip is 0.34 mm to create a globular stopper) was introduced into the internal carotid artery and advanced until resistance was felt. The filament was removed after 1 hr. Core body temperature was maintained at 37 ± 0.5°C on the heating pad. A total of 160 rats were divided into two groups; each group included four subgroups (each subgroup consisted of 20 rats): non-I/R (sham) group, vehicle-treated group, 8-O-acetyl shanzhiside methylester (ND01) 5 mg/kg group and 8-O-acetyl shanzhiside methylester (ND01) 10 mg/kg group. After 23 hr of reperfusion, all animals were administered an intravenous bolus injection (i.v., via the tail) of the corresponding drug daily for 14 days. The sham and vehicle-treated rats were both given saline. At 7 and 14 days, the first group of animals was used to evaluate nerve behaviour, then to measure permeability of the blood-brain barrier; each subgroup consisted of eight rats. At 7 and 14 days, the second group of animals was used to analyse Western blots, microvasculature density and immunohistochemical staining. Four rats of each subgroup were used to analyse Western blot in ischaemic brain core; the next four rats of each subgroup were used to detect microvasculature density and immunohistochemical staining.[3] |
| 参考文献 |
|
| 其他信息 |
8-O-Acetyl shanzhiside methyl ester has been reported in Barleria lupulina, Lamium garganicum, and other organisms with data available.
In the present study, we observed that 8-O-acetyl shanzhiside methylester (ND01) significantly improved brain injury and did not reduce blood glucose in diabetic rats subjected to cerebral I/R challenge, indicating that ND01 has an immediate neuroprotective effect but not by reducing blood glucose. Infarct size is the important indicator of the pathophysiology to evaluate the efficacy of cerebral ischaemia 19. Our results (fig. 3) revealed that 8-O-acetyl shanzhiside methylester (ND01) treatment produced significant reduction in the cerebral infarct volume in diabetic cerebral I/R rats even delayed administration at 2 and 4 hr after I/R. Neuronal degeneration and necrosis have been found to be correlated with deficits in behavioural disturbance, and behavioural assessment may reveal effectiveness 20. The present study showed that neurological scores were reduced by treatment with ND01 (fig. 3). NeuN is a sensitive marker for injured neuron early after ischaemic challenge 21, and our data (fig. 4) demonstrate that ND01 at a dose of 40 mg/kg attenuated the decrease in NeuN-immunopositive neurones in ischaemic cerebral cortex at 23 hr and 14 days after I/R. This indicates that ND01 has potential beneficial effects in the treatment of cerebral ischaemia. [1] The update of the Stroke Therapy Academic Industry Roundtable Preclinical Recommendations 22 lists potential reasons for failures in translating efficacious preclinical findings into successful clinical trial outcome. Consequently, we investigate the therapeutic time-window and the long-term efficacy of 8-O-acetyl shanzhiside methylester (ND01) in the cerebral I/R diabetic rats, and our data demonstrate that 8-O-acetyl shanzhiside methylester (ND01) exerted potent and long-term neuroprotective effects with a favourable therapeutic time-window. [1] Stroke triggers an inflammatory reaction that progresses for hours after the onset of the stroke, and this inflammation plays a central role in the pathogenesis of neuronal injury in ischaemic stroke, especially in diabetic stroke. Inflammatory reactions contribute to the late stages of ischaemic injury and worsened neurological outcome through the multiple mechanisms. DM is also an inflammatory disease. The present study indicates that 8-O-acetyl shanzhiside methylester (ND01) had significant anti-inflammatory effects (table 2 and fig. 5), and treatment with ND01 especially provides long-term benefits for neuronal functional recovery after cerebral I/R (table 1). This suggests that the neuroprotective effects of ND01 might be due to blocking of the inflammatory response. [1] MPO is considered an index of neutrophil infiltration and highly expressed cerebral ischaemia at 24 hr. It shows that a significant correlation between neutrophil infiltration and infarct formation exists in a model of cerebral ischaemia. Our results show that ND01 reduced MPO activity in diabetic ischaemic cerebral tissue. This suggests that the neuroprotective effects of ND01 might be due to blocking of neutrophil infiltration (table 2). [1] BBB permeability is significantly increased in diabetes due to diabetes-induced damage to the BBB function and/or due to the immature nature of the newly formed vessel. BBB breakdown also occurs in early-phase (within 24 hr) of cerebral ischaemia. Our data demonstrate that ND01 improved diabetic cerebral I/R injury by attenuating BBB breakdown. [1] NF-κB activation is associated with the phosphorylation of IκB-α and NF-κB in ischaemic cerebral tissue. Reduction in NF-κB activation can protect brain from activation of NF-κB-dependent genes. HMGB-1 is a novel player in the ischaemic brain. Meanwhile, diabetes significantly increased HMGB levels and induced worse functional outcome after stroke compared with non-diabetic MCAO rats. All those data show HMGB-1 plays a key role in diabetic stroke. The HMGB-1 signalling involves the activation of NF-κB, an inhibitor of NF-κB kinase essential for HMGB-1. Suppression the release of HMGB-1 in astrocytes leads to the attenuation of neuroinflammation and prevents the necrosis of ischaemic astrocytes and NF-κB expression 34. Inhibition of the up-regulation of HMGB-1 and NF-κB at the early stage brings great benefits to the diabetic cerebral ischaemia. Based on the above findings, we explored the anti-inflammatory properties of 8-O-acetyl shanzhiside methylester (ND01) in diabetic cerebral ischaemia and further studied the potential mechanisms. The up-regulation of HMGB-1 and NF-κB is significantly suppressed by 8-O-acetyl shanzhiside methylester (ND01). These results suggest that suppressing HMGB-1 and NF-κB expressions participated in the neuroprotection of ND01 against diabetic cerebral ischaemic damage. Therefore, we believe that the protective effects of ND01 might be due to suppression of the inflammatory cascades through HMGB-1-dependent NF-κB signalling pathway. [1] In summary, the results of the current study suggest that 8-O-acetyl shanzhiside methylester (ND01) exhibits significant neuroprotective effects during diabetic cerebral I/R injury, including attenuation of BBB breakdown, decrease in the infarct volume, alleviation of cerebral damage, reduction in HMGB-1 expression, phosphorylated IκB-α and NF-κB expression in ischaemic brain tissue. These effects of ND01 are correlated with inhibition of the inflammatory response. These findings point to a therapeutic potential for ND01 as a useful anti-inflammatory leads compound in early diabetic cerebral I/R injury. |
| 分子式 |
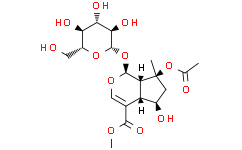
C₁₉H₂₈O₁₂
|
|---|---|
| 分子量 |
448.42
|
| 精确质量 |
448.158
|
| CAS号 |
57420-46-9
|
| 相关CAS号 |
57420-46-9
|
| PubChem CID |
162823
|
| 外观&性状 |
White to off-white solid
|
| 密度 |
1.52 g/cm3
|
| 沸点 |
634.2±55.0 °C at 760 mmHg
|
| 闪点 |
220.0±25.0 °C
|
| 蒸汽压 |
0.0±4.2 mmHg at 25°C
|
| 折射率 |
1.594
|
| LogP |
-2.76
|
| tPSA |
181.44
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
12
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
31
|
| 分子复杂度/Complexity |
725
|
| 定义原子立体中心数目 |
10
|
| SMILES |
O(C(C([H])([H])[H])=O)[C@@]1(C([H])([H])[H])C([H])([H])[C@]([H])([C@]2([H])C(C(=O)OC([H])([H])[H])=C([H])O[C@]([H])([C@]12[H])O[C@@]1([H])[C@@]([H])([C@]([H])([C@@]([H])([C@@]([H])(C([H])([H])O[H])O1)O[H])O[H])O[H])O[H]
|
| InChi Key |
ARFRZOLTIRQFCI-NGQYDJQZSA-N
|
| InChi Code |
InChI=1S/C19H28O12/c1-7(21)31-19(2)4-9(22)11-8(16(26)27-3)6-28-17(12(11)19)30-18-15(25)14(24)13(23)10(5-20)29-18/h6,9-15,17-18,20,22-25H,4-5H2,1-3H3/t9-,10-,11+,12-,13-,14+,15-,17+,18+,19+/m1/s1
|
| 化学名 |
methyl (1S,4aS,5R,7S,7aS)-7-acetyloxy-5-hydroxy-7-methyl-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4a,5,6,7a-tetrahydro-1H-cyclopenta[c]pyran-4-carboxylate
|
| 别名 |
8-O-Acetyl shanzhiside methyl ester; 8-O-Acetyl shanzhiside methyl ester; 8-O-Acetylshanzhiside methyl ester; methyl (1S,4aS,5R,7S,7aS)-7-acetyloxy-5-hydroxy-7-methyl-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4a,5,6,7a-tetrahydro-1H-cyclopenta[c]pyran-4-carboxylate; 5beta-Dihydro Finasteride; Cyclopenta[c]pyran-4-carboxylic acid,7-(acetyloxy)-1-(b-D-glucopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-5-hydroxy-7-methyl-, methyl ester, (1S,4aS,5R,7S,7aS)-; Umbroside; Barlerin
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~100 mg/mL (~223.0 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.58 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.58 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.58 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2301 mL | 11.1503 mL | 22.3005 mL | |
| 5 mM | 0.4460 mL | 2.2301 mL | 4.4601 mL | |
| 10 mM | 0.2230 mL | 1.1150 mL | 2.2301 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。