| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
JAK2 (IC50 = 5.7 nM); JAK1 (IC50 = 5.9 nM); Tyk2 (IC50 = 53nM); JAK3 (IC50 = 560nM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
在细胞实验中,Baricitinib 磷酸盐(INCB028050 磷酸盐)是 JAK 信号传导和功能的强抑制剂。 Baricitinib 的 IC50 值分别为 44 nM 和 40 nM,可防止 IL-6 刺激的经典底物 STAT3 (pSTAT3) 磷酸化以及随后在 PBMC 中产生趋化因子 MCP-1。 INCB028050 还可抑制分离的初始 T 细胞中由 IL-23 激活的 pSTAT3 (IC50=20 nM)。值得注意的是,在 IC50 值为 50 nM 时,这种抑制作用阻止了 Th17 细胞(具有可观察到的炎症和致病特征的辅助 T 细胞的一个子集)产生两种致病性细胞因子(IL-17 和 IL-22)。另一方面,当在高达 10 μM 的浓度下进行评估时,结构相似但无效的 JAK1/2 抑制剂 INCB027753 和 INCB029843 在任何这些测定系统中都没有明显的作用[1]。
|
||
| 体内研究 (In Vivo) |
与相比,磷酸巴瑞克替尼(INCB028050磷酸盐)治疗在治疗的前两周,在剂量为1 mg/kg时可抑制后爪体积的增加50%,在剂量为3或10 mg/kg时可抑制后爪体积的增加95%以上到车辆。对于肿胀明显改善的动物来说,抑制 >100% 是可行的,因为基线爪体积测量是在治疗第 0 天在具有明显疾病症状的动物中获得的[1]。与给予载体对照的小鼠相比,用巴瑞克替尼(0.7 mg/天)治疗的小鼠通过 H&E 染色测量显示炎症显着减少、CD8 浸润减少以及 I 类和 II 类 MHC 表达减少。与用载体对照治疗的小鼠相比,用巴瑞克替尼治疗的小鼠中 CD8+NKG2D+ 细胞的数量显着减少,CD8+NKG2D+ 细胞是人类和动物斑秃 (AA) 的重要疾病效应细胞。
|
||
| 酶活实验 |
使用具有重组表位标记的激酶结构域(JAK1837-1142;JAK2828-1132;JAK3718-1124;Tyk2873-1187)或全长酶(cMET和Chk2)和肽底物的均匀时间分辨荧光测定法进行酶测定。在测定缓冲液中使用或不使用测试化合物(11点稀释)、JAK、cMET或Chk2酶、500nM(对于Chk2为100nM)肽、ATP(在每种激酶特异性的Km或1mM)和2.0%DMSO进行每种酶反应。计算出的IC50值是抑制50%荧光信号所需的化合物浓度。使用200 nM的标准条件在Cerep进行额外的激酶测定。测试的酶包括:Abl、Akt1、AurA、AurB、CDC2、CDK2、CDK4、CHK2、c-kit、EGFR、EphB4、ERK1、ERK2、FLT-1、HER2、IGF1R、IKKα、IKKβ、JNK1、Lck、MEK1、p38α、p70S6K、PKA、PKCα、Src和ZAP70。[1]
|
||
| 细胞实验 |
细胞测定[1]
通过leukapetheresis和Ficoll-Hypaque离心分离人PBMC。为了测定IL-6诱导的MCP-1的产生,在存在或不存在各种浓度的INCB028050的情况下,将PBMC以3.3×105个细胞/孔的速度接种在RPMI 1640+10%FCS中。在室温下与化合物预孵育10分钟后,通过向每个孔中加入10ng/ml人重组IL-6来刺激细胞。将细胞在37°C、5%CO2下孵育48小时。采集上清液并通过ELISA分析人MCP-1的水平。INCB028050抑制IL-6诱导的MCP-1分泌的能力被报道为50%抑制所需的浓度(IC50)。使用Cell Titer Glo在标准测定条件下在3天内进行Ba/F3-TEL-JAK3细胞的增殖。 为了测定IL-23诱导的IL-17和IL-22,将PBMC维持在补充有10%FBS、2mM l-谷氨酰胺、100μg/ml链霉素和100U/ml青霉素的RPMI 1640培养基中。通过用抗CD3和抗CD28 Abs培养来活化T细胞。2天后,洗涤细胞并用IL-23(100ng/ml)、IL-2(10ng/ml)和各种浓度的INCB028050再培养。将细胞在37°C下再孵育4天,然后收集上清液,并通过ELISA测量IL-17和IL-22的分泌。INCB028050抑制IL-23诱导的IL-17和IL-22分泌的能力被报道为50%抑制所需的浓度(IC50)。 磷酸-STAT3分析[1] 分离的细胞。[1] 为了分析人PBMC或PHA刺激的T细胞中的磷酸化-STAT3,在用不同浓度的INCB028050预孵育10−15分钟并用IL-6(100 ng/ml)、IL-12(20 ng/ml)或IL-23(100 ng/ml)刺激细胞15分钟后制备细胞提取物。然后通过使用磷酸化-STAT3特异性ELISA分析提取物的磷酸化STAT3。 全血。[1] 将从大鼠抽取的血液收集到肝素化管中,然后等分到微量离心管中(每个样品0.3ml)。在刺激实验中,在37°C下用人IL-6(100 ng/ml)刺激15分钟之前,加入不同浓度的INCB028050 10分钟。使用低渗条件裂解RBCs。然后将WBC快速成丸并裂解以制备总的细胞提取物。使用磷酸化STAT3特异性ELISA分析提取物中的磷酸化STAT3。在INCB028050给药后的不同时间从给药INCB02805的动物中抽取血液,并如上所述进行处理。 |
||
| 动物实验 |
|
||
| 参考文献 |
|
||
| 其他信息 |
Inhibiting signal transduction induced by inflammatory cytokines offers a new approach for the treatment of autoimmune diseases such as rheumatoid arthritis. Kinase inhibitors have shown promising oral disease-modifying antirheumatic drug potential with efficacy similar to anti-TNF biologics. Direct and indirect inhibition of the JAKs, with small molecule inhibitors like CP-690,550 and INCB018424 or neutralizing Abs, such as the anti-IL6 receptor Ab tocilizumab, have demonstrated rapid and sustained improvement in clinical measures of disease, consistent with their respective preclinical experiments. Therefore, it is of interest to identify optimized JAK inhibitors with unique profiles to maximize therapeutic opportunities. INCB028050 is a selective orally bioavailable JAK1/JAK2 inhibitor with nanomolar potency against JAK1 (5.9 nM) and JAK2 (5.7 nM). INCB028050 inhibits intracellular signaling of multiple proinflammatory cytokines including IL-6 and IL-23 at concentrations <50 nM. Significant efficacy, as assessed by improvements in clinical, histologic and radiographic signs of disease, was achieved in the rat adjuvant arthritis model with doses of INCB028050 providing partial and/or periodic inhibition of JAK1/JAK2 and no inhibition of JAK3. Diminution of inflammatory Th1 and Th17 associated cytokine mRNA levels was observed in the draining lymph nodes of treated rats. INCB028050 was also effective in multiple murine models of arthritis, with no evidence of suppression of humoral immunity or adverse hematologic effects. These data suggest that fractional inhibition of JAK1 and JAK2 is sufficient for significant activity in autoimmune disease models. Clinical evaluation of INCB028050 in RA is ongoing.[1]
Background: Alopecia areata (AA) is an autoimmune disease resulting in hair loss with devastating psychosocial consequences. Despite its high prevalence, there are no FDA-approved treatments for AA. Prior studies have identified a prominent interferon signature in AA, which signals through JAK molecules.[2] Methods: A patient with AA was enrolled in a clinical trial to examine the efficacy of baricitinib, a JAK1/2 inhibitor, to treat concomitant CANDLE syndrome. In vivo, preclinical studies were conducted using the C3H/HeJ AA mouse model to assess the mechanism of clinical improvement by baricitinib.[2] Findings: The patient exhibited a striking improvement of his AA on baricitinib over several months. In vivo studies using the C3H/HeJ mouse model demonstrated a strong correlation between resolution of the interferon signature and clinical improvement during baricitinib treatment.[2] Interpretation: Baricitinib may be an effective treatment for AA and warrants further investigation in clinical trials.[2] Keywords: Alopecia areata; Autoimmune disease; Autoinflammatory; Baricitinib; CANDLE syndrome; Gene expression profiling; Interferon gamma; JAK inhibitor.[2] |
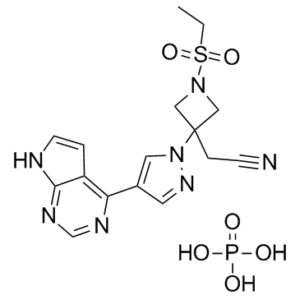
| 分子式 |
C16H20N7O6PS
|
|---|---|
| 分子量 |
469.41
|
| 精确质量 |
469.093
|
| 元素分析 |
C, 40.94; H, 4.29; N, 20.89; O, 20.45; P, 6.60; S, 6.83
|
| CAS号 |
1187595-84-1
|
| 相关CAS号 |
Baricitinib;1187594-09-7
|
| PubChem CID |
44231848
|
| 外观&性状 |
Typically exists as white to yellow solids at room temperature
|
| LogP |
1.185
|
| tPSA |
216.51
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
31
|
| 分子复杂度/Complexity |
728
|
| 定义原子立体中心数目 |
0
|
| SMILES |
S(C([H])([H])C([H])([H])[H])(N1C([H])([H])C(C([H])([H])C#N)(C1([H])[H])N1C([H])=C(C2=C3C([H])=C([H])N([H])C3=NC([H])=N2)C([H])=N1)(=O)=O.P(=O)(O[H])(O[H])O[H]
|
| InChi Key |
FBPOWTFFUBBKBB-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C16H17N7O2S.H3O4P/c1-2-26(24,25)22-9-16(10-22,4-5-17)23-8-12(7-21-23)14-13-3-6-18-15(13)20-11-19-14;1-5(2,3)4/h3,6-8,11H,2,4,9-10H2,1H3,(H,18,19,20);(H3,1,2,3,4)
|
| 化学名 |
(1-(Ethylsulfonyl)-3-(4-(7H-pyrrolo(2,3-d)pyrimidin-4-yl)-1H-pyrazol-1-yl)azetidin-3-yl)ethanenitrile phosphate
|
| 别名 |
trade name Olumiant; LY3009104 phosphate; LY-3009104; LY 3009104; INCB028050 phosphate; INCB-028050; INCB 028050; Baricitinib phosphate;Baricitinib phosphate; 1187595-84-1; Baricitinib (phosphate); Baricitinib phosphate salt; INCB-28050; XIB47S8NNB;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.43 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.08 mg/mL (4.43 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.43 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 0.5% CMC+0.25% Tween 80:30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1303 mL | 10.6517 mL | 21.3033 mL | |
| 5 mM | 0.4261 mL | 2.1303 mL | 4.2607 mL | |
| 10 mM | 0.2130 mL | 1.0652 mL | 2.1303 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01398475 | Completed Has Results | Drug: LY3009104 | Chronic Inflammatory Disorder Arthritis, Rheumatoid |
Eli Lilly and Company | July 2011 | Phase 1 |
| NCT05452564 | Recruiting | Drug: Baricitinib 2 MG Oral Tablet | Human Immunodeficiency Virus | William Tyor | May 18, 2023 | Phase 2 |
| NCT04640168 | Completed Has Results | Drug: Baricitinib Drug: Dexamethasone | Recurrent Glioma Refractory Glioma |
National Institute of Allergy and Infectious Diseases (NIAID) |
December 2, 2020 | Phase 3 |
Cellular activity of INCB028050.J Immunol.2010 May 1;184(9):5298-307. |
Anti-inflammatory and DMARD activity of once daily INCB028050 in rats with established disease in the adjuvant arthritis model.J Immunol.2010 May 1;184(9):5298-307. |
Suppression of delayed-type hypersensitivity by INCB028050.J Immunol.2010 May 1;184(9):5298-307. |
INCB028050 is efficacious and well tolerated independently of effects on humoral immunity.J Immunol.2010 May 1;184(9):5298-307. |
INCB028050 improves clinical and histologic signs of disease in the murine CIA model. |