| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| 100g |
|
||
| 200g |
|
||
| Other Sizes |
|

| 靶点 |
COX-1 (IC50 = 27.75 μM); COX-2 (IC50 = 1.17 mM)
|
|---|---|
| 体外研究 (In Vitro) |
在人关节软骨细胞中,阿司匹林抑制 COX-1 和 COX-2,IC50 值分别为 3.57 μM 和 29.3 μM [2]。阿司匹林通过乙酰化 COX-1 的丝氨酸 530,抑制血小板聚集并防止血小板中血栓素 A 的产生 [3]。通过与 CCAAT/增强子结合蛋白 β (C/EBPbeta) 及其在 COX-2 启动子/增强子上的相应位置相互作用,阿司匹林抑制 COX-2 蛋白的表达 [3]。在感染 HIV 的 T 细胞中,阿司匹林以 NF-κB 依赖性方式阻断 lgκ 增强子和长末端重复序列 (LTR) 的转录 [4]。阿司匹林释放线粒体细胞色素c,触发神经酰胺途径,激活半胱天冬酶,并激活p38 MAP激酶。
|
| 体内研究 (In Vivo) |
在动物模型中,阿司匹林可用于创建胃肠道溃疡模型。患有酵母热的雄性成年大鼠对阿司匹林(5-150 mg/kg,口服,一次)有显着反应[3-4]。
阿司匹林是一种常用的非甾体抗炎药,但长期使用会损伤胃黏膜。本研究旨在评估螺旋藻对阿司匹林诱导的白化小鼠胃溃疡的改善作用。口服阿司匹林(500mg/kg bw)诱发胃溃疡。诱导胃溃疡后,口服螺旋藻(250和500 mg/kg bw)3天。螺旋藻通过改善胃组织的总体形态、组织学和粘膜层,增加内源性酶和非酶抗氧化剂(还原型谷胱甘肽、谷胱甘肽过氧化物酶、谷胱甘肽还原酶、超氧化物歧化酶和过氧化氢酶)和细胞保护标志物(COX-1),以及减轻脂质过氧化标志物(丙二醛)和炎症介质(TNF-α、COX-2和NO)的组织水平,改善了阿司匹林诱导的胃溃疡。总之,螺旋藻通过减轻氧化应激和炎症,对阿司匹林诱导的胃损伤具有治疗潜力。[4] 胃溃疡模型的建立[4] 将小鼠放置在带有宽网活动地板的代谢笼中,以避免食粪,这会影响胃溃疡的诱导。动物禁食24小时以排空胃中的食物并增加胃酸水平,从而促进阿司匹林给药后的胃损伤。实验前一小时,水也被扣留了。单次口服乙酰水杨酸(500mg/kg体重)诱导胃黏膜损伤。 Spirulina对胃溃疡的治疗[4] 动物被随机分为五组(n=7)。第1组接受车辆,作为阴性对照组。第2组通过胃管法接Spirulina(500 mg/kg bw)治疗三天,作为螺旋藻对照组。第3组接受单次口服阿司匹林,剂量为500mg/kg bw,悬浮在水中,作为溃疡对照组。第4组和第5组服用阿司匹林,然后分别以250和500mg/kg b.w的剂量用螺旋藻治疗三天。 |
| 酶活实验 |
激酶活性测定。[2]
从转染细胞制备裂解物(200μg蛋白质),并在4°C下与抗体(抗Flag(M2)、抗HA(12CA5)或抗Myc)孵育1小时,加入20μl蛋白A-琼脂糖1小时。在广泛洗涤免疫沉淀物后,按照所述进行激酶测定11。对于体外激酶测定,在激酶反应前,在4°C下将阿司匹林加入洗涤过的免疫沉淀物中30分钟。混合物经过SDS-PAGE和放射自显影,并通过磷光分析进行定量。 阿司匹林IC50的计算。[2] 为了测定内源性IKK活性,用针对IKK-α的兔多克隆抗体免疫沉淀细胞裂解物(200μg蛋白质),该抗体免疫沉淀IKK-α/IKK-β异二聚体,然后测定激酶活性。通过杆状病毒表达产生多组氨酸和Flag标记的IKK-α和IKK-β蛋白,并用镍琼脂糖层析纯化。使用12CA5单克隆抗体对纯化的蛋白质(500μg)进行免疫沉淀,分为10个相等的组分,每个组分在冰上用不同浓度的阿司匹林或水杨酸钠处理30分钟。然后用磷酸受体测定和定量激酶活性;计算阿司匹林对激酶活性的抑制作用,并绘制阿司匹林浓度图。 IKK与14C-水杨酸盐结合和14C-阿司匹林结合。[2] 从杆状病毒表达和纯化的IKK-α和IKK-β蛋白或用IKK-α或IKK-βcDNA转染的细胞中分离出的蛋白质(200μg)用表位特异性单克隆抗体免疫沉淀,然后用500μl结合缓冲液孵育,该缓冲液含有100 mM NaCl、50 mM Tris、pH 7.5、10 mg ml-1 BSA、蛋白酶抑制剂和2μCi乙酰水杨酸14C羧酸或[7-14C]水杨酸(40-60 mCi mmol−1)。将500倍摩尔过量(36 mM)的阿司匹林、水杨酸钠、吲哚美辛或ATP加入到每个免疫沉淀物中,并在4°C下孵育30分钟。然后用结合缓冲液广泛洗涤免疫沉淀物,并通过β计数定量结合的14C-水杨酸盐或14C-阿司匹林的量。免疫沉淀物也与20%TCA一起孵育,通过离心分离沉淀物并溶解在1M NaOH中。通过β计数定量蛋白质沉淀物中14C-阿司匹林和14C-水杨酸的量。10或20μg COX-1蛋白用于与IKK蛋白的结合反应。 转录因子核因子κB(NF-kappa B)对于参与炎症和感染的多种细胞和病毒基因的诱导表达至关重要,包括白细胞介素-1(IL-1)、IL-6和粘附分子。抗炎药水杨酸钠和阿司匹林抑制了NF-κB的激活,这进一步解释了这些药物的作用机制。这种抑制阻止了NF-κB抑制剂IκB的降解,因此NF-κB保留在细胞质中。水杨酸钠和阿司匹林还抑制了转染T细胞中Igκ增强子和人类免疫缺陷病毒(HIV)长末端重复序列(LTR)的NF-κB依赖性转录。[1] NF-kappaB包含一个细胞转录因子家族,参与调节炎症反应的各种细胞基因的诱导表达。NF-kappaB被抑制性蛋白I(kappa)B隔离在细胞质中,I(kappaB)B被称为IKK的细胞激酶复合物磷酸化。IKK由两种激酶组成,IKKα和IKKβ,它们磷酸化I(κ)B,导致其降解并将NF-κB转运到细胞核。当细胞暴露于细胞因子TNF-α或通过细胞激酶MEKK1和NIK的过表达时,IKK激酶活性受到刺激。在这里,我们证明抗炎药阿司匹林和水杨酸钠在体外和体内特异性抑制IKKβ活性。阿司匹林和水杨酸钠抑制的机制是由于这些药物与IKKβ结合以减少ATP结合。我们的结果表明,阿司匹林和水杨酸盐的抗炎特性部分是通过其对IKKβ的特异性抑制来介导的,从而阻止NF-kappaB激活参与炎症反应发病机制的基因。[2] |
| 细胞实验 |
细胞培养和转染。[2]
COS和HeLa细胞转染Fugene 6;用DEAE-葡聚糖转染Jurkat细胞。转染后24小时,在阿司匹林(5 mM)、水杨酸钠(5 mmol)、地塞米松(10μM)或吲哚美辛(25μM)存在或不存在的情况下收集细胞。HIV1-LTR-CAT和E3-CAT报告子构建11,20,并描述了标记为IKK-α(HA)、IKK-β(Flag)、NIK(c-Myc)、Tax、MEKK1、p38(HA),SAPK(Myc)和Erk2(Myc,Myc)的表位11,21,22,22,23,24。在收集前10分钟向细胞中加入TNF-α(20 ng ml-1)以刺激IKK激酶活性,并在转染后20小时加入TNF-α以检测NFκB介导的基因表达。转染SAPK和p38 cDNA的细胞在收集前用茴香霉素(10μg ml-1)处理30分钟;用TPA(12-O-十四烷酰佛波醇-13-乙酸酯;50 ng ml-1)预处理转染了Erk2 cDNA的细胞30 min24,以激活这些激酶。将阿司匹林(乙酰水杨酸)和水杨酸钠(Sigma)溶解在0.05 M Tris-HCl中,制备1.0 M储备溶液;地塞米松和毛喉素的制备方法如下18所述。将细胞上清液施加到C18微柱上,并使用ELISA试剂盒检测前列腺素。 |
| 动物实验 |
Animal/Disease Models: Male albino Charles River rats (200-250 g, 8 animals/group, fever was induced by 20 ml/kg of a 20 % aqueous suspension of brewer's yeast which was injected SC in the back below the nape of the neck) [7]
Doses: 5, 25, 50, 100 and 150 mg/kg Route of Administration: PO, once Experimental Results: Produced a statistically significant decrease of 0.23 ℃ at 15 min post-drug at the dose of 150 mg/kg. Antipyretic effect gradually increased in magnitude until a peak effect of 1.96 ℃ was reached at 120 min post-drug. The ED50 of aspirin was found to be 10.3 mg/kg with confidence limits of 1.8-23.0 mg/kg. The antipyretic response to aspirin is dependent on the dose of the compound administered. Induction of gastric ulcer[4] Mice were placed in metabolic cages with raised floors of wide mesh to avoid coprophagy, which affects the induction of gastric ulcer. The animals were fasted for 24 h to empty the stomach of food and increase the gastric acid level, thereby facilitating gastric injury upon aspirin administration. One hour before the experiments, water was also withheld. Gastric mucosal injury was induced by a single oral dose of acetyl salicylic acid (500 mg/kg body weight). Experimental design[4] The animals were randomly assigned to five groups (n = 7). Group 1 received the vehicle and served as negative control group. Group 2 received Spirulina (500 mg/kg bw) for three days by a gastric gavage, and served as Spirulina-control group. Group 3 received a single oral dose of aspirin at a dose of 500 mg/kg bw suspended in water, and served as ulcer-control group. Group 4 and 5 were given aspirin, then treated with Spirulina at dose 250 and 500 mg/kg b.w for three days, respectively. |
| 参考文献 |
[1]. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science.1994 Aug 12;265(5174):956-9;
[2]. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature.1998 Nov 5;396(6706):77-80. [3]. Antipyretic testing of aspirin in rats. Toxicol Appl Pharmacol 1972 Aug;22(4):672-5. [4]. Spirulina ameliorates aspirin-induced gastric ulcer in albino mice by alleviating oxidative stress and inflammation. Biomed Pharmacother. 2019 Jan:109:314-321. |
| 其他信息 |
Acetylsalicylic acid appears as odorless white crystals or crystalline powder with a slightly bitter taste. (NTP, 1992)
National Toxicology Program, Institute of Environmental Health Sciences, National Institutes of Health (NTP). 1992. National Toxicology Program Chemical Repository Database. Research Triangle Park, North Carolina.
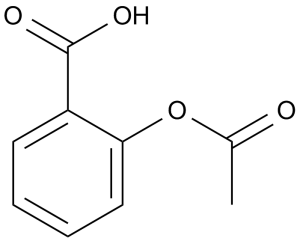
Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an acetoxy group. A non-steroidal anti-inflammatory drug with cyclooxygenase inhibitor activity. It has a role as a non-steroidal anti-inflammatory drug, a non-narcotic analgesic, a platelet aggregation inhibitor, an antipyretic, a cyclooxygenase 2 inhibitor, a cyclooxygenase 1 inhibitor, a prostaglandin antagonist, a teratogenic agent, an anticoagulant, a plant activator, an EC 1.1.1.188 (prostaglandin-F synthase) inhibitor, a drug allergen and a geroprotector. It is a member of benzoic acids, a member of salicylates and a member of phenyl acetates. It is functionally related to a salicylic acid. It is a conjugate acid of an acetylsalicylate. Also known as Aspirin, acetylsalicylic acid (ASA) is a commonly used drug for the treatment of pain and fever due to various causes. Acetylsalicylic acid has both anti-inflammatory and antipyretic effects. This drug also inhibits platelet aggregation and is used in the prevention of blood clots stroke, and myocardial infarction (MI). Interestingly, the results of various studies have demonstrated that long-term use of acetylsalicylic acid may decrease the risk of various cancers, including colorectal, esophageal, breast, lung, prostate, liver and skin cancer. Aspirin is classified as a non-selective cyclooxygenase (COX) inhibitor and is available in many doses and forms, including chewable tablets, suppositories, extended release formulations, and others. Acetylsalicylic acid is a very common cause of accidental poisoning in young children. It should be kept out of reach from young children, toddlers, and infants. Aspirin is a Nonsteroidal Anti-inflammatory Drug and Platelet Aggregation Inhibitor. The mechanism of action of aspirin is as a Cyclooxygenase Inhibitor. The physiologic effect of aspirin is by means of Decreased Prostaglandin Production and Decreased Platelet Aggregation. Aspirin or acetylsalicylic acid is perhaps the most commonly used analgesic and antipyretic medication worldwide, having been in clinical use for over 100 years. Aspirin can cause several forms of liver injury: in high doses, aspirin can cause moderate to marked serum aminotransferase elevations occasionally with jaundice or signs of liver dysfunction, and in lower doses in susceptible children with a febrile illness aspirin can lead to Reye syndrome. View More
Aspirin is an orally administered non-steroidal antiinflammatory agent. Acetylsalicylic acid binds to and acetylates serine residues in cyclooxygenases, resulting in decreased synthesis of prostaglandin, platelet aggregation, and inflammation. This agent exhibits analgesic, antipyretic, and anticoagulant properties.
Drug Indication **Pain, fever, and inflammation** Acetylsalicylic acid (ASA), in the regular tablet form (immediate-release), is indicated to relieve pain, fever, and inflammation associated with many conditions, including the flu, the common cold, neck and back pain, dysmenorrhea, headache, tooth pain, sprains, fractures, myositis, neuralgia, synovitis, arthritis, bursitis, burns, and various injuries. It is also used for symptomatic pain relief after surgical and dental procedures. The _extra strength_ formulation of acetylsalicylic acid is also indicated for the management migraine pain with photophobia (sensitivity to light) and phonophobia (sensitivity to sound). **Other indications** ASA is also indicated for various other purposes, due to its ability to inhibit platelet aggregation. These include: Reducing the risk of cardiovascular death in suspected cases of myocardial infarction (MI). Reducing the risk of a first non-fatal myocardial infarction in patients, and for reducing the risk of morbidity and mortality in cases of unstable angina and in those who have had a prior myocardial infarction. For reducing the risk of transient ischemic attacks (TIA) and to prevent atherothrombotic cerebral infarction (in conjunction with other treatments). For the prevention of thromboembolism after hip replacement surgery. For decreasing platelet to platelet adhesion following carotid endarterectomy, aiding in the prevention of transient ischemic attacks (TIA). Used for patients undergoing hemodialysis with a silicone rubber arteriovenous cannula inserted to prevent thrombosis at the insertion site. **Important note regarding use of the extended-release formulation** In the setting of acute myocardial infarction, or before percutaneous interventions, the extended-release form of acetylsalicylic acid should not be used. Use immediate-release formulations in scenarios requiring rapid onset of action. The extended-release form is taken to decrease the incidence of mortality and myocardial infarction (MI) for individuals diagnosed with chronic coronary artery disease (CAD), including patients with previous myocardial infarction (MI) or unstable angina or with chronic stable angina. Additionally, the extended-release form is used to decrease the risk of death and recurrent episodes of stroke in patients with a history of stroke or TIA. Therapeutic Uses Anti-Inflammatory Agents, Non-Steroidal; Cyclooxygenase Inhibitors; Fibrinolytic Agents; Platelet Aggregation Inhibitors National Library of Medicine's Medical Subject Headings online file (MeSH, 1999) Salicylates are indicated to relieve myalgia, musculoskeletal pain, and other symptoms of nonrheumatic inflammatory conditions such as athletic injuries, bursitis, capsulitis, tendinitis, and nonspecific acute tenosynovitis. /Included in US product labeling/ Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2574 Salicylates are indicated for the symptomatic relief of acute and chronic rheumatoid arthritis, juvenile arthritis, osteoarthritis, and related rheumatic diseases. Aspirin is usually the first agent to be used and may be the drug of choice in patients able to tolerate prolonged therapy with high doses. These agents do not affect the progressive course of rheumatoid arthritis. Concurrent treatment with a glucocorticoid or a disease-modifying antirheumatic agent may be needed, depending on the condition being treated and patient response. /Included in US product labeling/ Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2574 Salicylates are also used to reduce arthritic complications associated with systemic lupus erythematosus. /Salicylates; NOT included in US product labeling/ Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2574 Drug Warnings Aspirin use may be associated with the development of Reye's syndrome in children and teenagers with acute febrile illnesses, especially influenza and varicella. It is recommended that salicylate therapy not be initiated in febrile pediatric or adolescent patients until after the presence of such an illness has been ruled out. Also, it is recommended that chronic salicylate therapy in these patients be discontinued if a fever occurs, and not resumed until it has been determined that an illness that may predispose to Reye's syndrome is not present or has run its course. Other forms of salicylate toxicity may also be more prevalent in pediatric patients, especially children who have a fever or are dehydrated. Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2577 Especially careful monitoring of the serum salicylate concentration is recommended in pediatric patients with Kawasaki disease. Absorption of aspirin is impaired during the early febrile stage of the disease; therapeutic anti-inflammatory plasma salicylate concentrations may be extremely difficult to achieve. Also, as the febrile stage passes, absorption is improved; salicylate toxicity may occur if dosage is not readjusted. Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2577 Drug Idiosyncrasies ... SOME PEOPLE MANIFEST IDIOSYNCRASY IN FORM OF ALLERGIC SENSITIVITY TO SALICYLATES, ESP ASPIRIN, & MAY SUFFER FROM SERIOUS IF NOT FATAL ASTHMA AFTER INGESTION OF SINGLE 0.3 G DOSE. Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1049 Aspirin is a known respiratory and systemic allergen and can produce anaphylactic phenomena even after small doses. American Conference of Governmental Industrial Hygienists. Documentation of the TLV's and BEI's with Other World Wide Occupational Exposure Values. CD-ROM Cincinnati, OH 45240-1634 2007. An acute irritant to the gastric mucosa ... Ingestion of aspirin produce an increased tendency to bleed (increased clotting time) due to its interference with platelet aggregation. A normal, therapeutic dose ... 600 mg can produce these abnormalities for five days or longer. However, these toxic effects have also been demonstrated by ingestion of 150 mg, the smallest dose reported to have a pharmacologic effect. American Conference of Governmental Industrial Hygienists. Documentation of the TLV's and BEI's with Other World Wide Occupational Exposure Values. CD-ROM Cincinnati, OH 45240-1634 2007. Reported Fatal Dose The lethal dose of aspirin for an adult is probably in the region of 25 to 30 g but recovery has been achieved by appropriate treatment after the ingestion of twice or thrice this amount. Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 236 Maximum Drug Dose The usual dose of aspirin as an analgesic and antipyretic is 0.3 to 1 g, which may be repeated every 4 hours according to clinical needs, up to a maximum of 4 g daily. Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 240 Pharmacodynamics **Effects on pain and fever** Acetylsalicylic acid disrupts the production of prostaglandins throughout the body by targeting cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). Prostaglandins are potent, irritating substances that have been shown to cause headaches and pain upon injection into humans. Prostaglandins increase the sensitivity of pain receptors and substances such as histamine and bradykinin. Through the disruption of the production and prevention of release of prostaglandins in inflammation, this drug may stop their action at pain receptors, preventing symptoms of pain. Acetylsalicylic acid is considered an antipyretic agent because of its ability to interfere with the production of brain prostaglandin E1. Prostaglandin E1 is known to be an extremely powerful fever-inducing agent. **Effects on platelet aggregation** The inhibition of platelet aggregation by ASA occurs because of its interference with thromboxane A2 in platelets, caused by COX-1 inhibition. Thromboxane A2 is an important lipid responsible for platelet aggregation, which can lead to clot formation and future risk of heart attack or stroke. **A note on cancer prevention** ASA has been studied in recent years to determine its effect on the prevention of various malignancies. In general, acetylsalicylic acid is involved in the interference of various cancer signaling pathways, sometimes inducing or upregulating tumor suppressor genes. Results of various studies suggest that there are beneficial effects of long-term ASA use in the prevention of several types of cancer, including stomach, colorectal, pancreatic, and liver cancers. Research is ongoing. Absorption Absorption is generally rapid and complete following oral administration but absorption may be variable depending on the route, dosage form, and other factors including but not limited to the rate of tablet dissolution, gastric contents, gastric emptying time, and gastric pH. **Detailed absorption information** When ingested orally, acetylsalicylic acid is rapidly absorbed in both the stomach and proximal small intestine. The non-ionized acetylsalicylic acid passes through the stomach lining by passive diffusion. Ideal absorption of salicylate in the stomach occurs in the pH range of 2.15 - 4.10. Intestinal absorption of acetylsalicylic acid occurs at a much faster rate. At least half of the ingested dose is hydrolyzed to salicylic acid in the first-hour post-ingestion by esterases found in the gastrointestinal tract. Peak plasma salicylate concentrations occur between 1-2 hours post-administration. Route of Elimination Excretion of salicylates occurs mainly through the kidney, by the processes of glomerular filtration and tubular excretion, in the form of free salicylic acid, salicyluric acid, and, additionally, phenolic and acyl glucuronides. Salicylate can be found in the urine soon after administration, however, the entire dose takes about 48 hours to be completely eliminated. The rate of salicylate is often variable, ranging from 10% to 85% in the urine, and heavily depends on urinary pH. Acidic urine generally aids in reabsorption of salicylate by the renal tubules, while alkaline urine increases excretion. After the administration of a typical 325mg dose, the elimination of ASA is found to follow first order kinetics in a linear fashion. At high concentrations, the elimination half-life increases. Volume of Distribution This drug is distributed to body tissues shortly after administration. It is known to cross the placenta. The plasma contains high levels of salicylate, as well as tissues such as spinal, peritoneal and synovial fluids, saliva and milk. The kidney, liver, heart, and lungs are also found to be rich in salicylate concentration after dosing. Low concentrations of salicylate are usually low, and minimal concentrations are found in feces, bile, and sweat. Clearance The clearance rate of acetylsalicylic acid is extremely variable, depending on several factors. Dosage adjustments may be required in patients with renal impairment. The extended-release tablet should not be administered to patients with eGFR of less than 10 mL/min. The materno-fetal transfer of salicylic acid and its distribution in the fetal organism was investigated in women of early pregnancy. Acetylsalicylic acid was administered orally in a single dose or in repeated doses at different times before legal interruption. The mean passage rates were about 6-15%. They were independent of the maternal serum concentrations of salicylic acid. The distribution of salicylic acid on the fetal liver, intestine, kidneys, lungs and brain was different. All fetal organs (9th to 15th week of gestation) studied exhibit an acetylsalicylic acid-splitting esterase activity. The esterase activity of the fetal liver was about 30% of the hydrolytic activity of the adult liver. The esterase activity was mainly located in the 105 000 X g-supernatant of cell homogenates. PMID:6651815 Amon I et al; Biomed Biochim Acta 42 (7-8): 997-1004 (1983) Hazardous Substances Data Bank (HSDB) Approximately 80-100% of an oral dose of aspirin is absorbed from the GI tract. However, the actual bioavailability of the drug as unhydrolyzed aspirin is lower since aspirin is partially hydrolyzed to salicylate in the GI mucosa during absorption and on first pass through the liver. There are relatively few studies of the bioavailability of unhydrolyzed aspirin. In one study in which aspirin was administered IV and as an oral aqueous solution, it was shown that the solution was completely absorbed but only about 70% reached the systemic circulation as unhydrolyzed aspirin. In another study in which aspirin was administered IV and orally as capsules, only about 50% of the oral dose reached the systemic circulation as unhydrolyzed aspirin. There is some evidence that the bioavailability of unhydrolyzed aspirin from slowly absorbed dosage forms (e.g., enteric-coated tablets) may be substantially decreased. Food does not appear to decrease the bioavailability of unhydrolyzed aspirin or salicylate; however, absorption is delayed and peak serum aspirin or salicylate concentration may be decreased. There is some evidence that absorption of salicylate following oral administration may be substantially impaired or is highly variable during the febrile phase of Kawasaki disease. McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2036 Metabolism / Metabolites Acetylsalicylic acid is hydrolyzed in the plasma to salicylic acid. Plasma concentrations of aspirin following after administration of the extended-release form are mostly undetectable 4-8 hours after ingestion of a single dose. Salicylic acid was measured at 24 hours following a single dose of extended-release acetylsalicylic acid. Salicylate is mainly metabolized in the liver, although other tissues may also be involved in this process. The major metabolites of acetylsalicylic acid are salicylic acid, salicyluric acid, the ether or phenolic glucuronide and the ester or acyl glucuronide. A small portion is converted to gentisic acid and other hydroxybenzoic acids. Biological Half-Life The half-life of ASA in the circulation ranges from 13 - 19 minutes. Blood concentrations drop rapidly after complete absorption. The half-life of the salicylate ranges between 3.5 and 4.5 hours. 15 to 20 minutes (for intact molecule); rapidly hydrolyzed to salicylate. In breast milk (as salicylate): 3.8 to 12.5 hours (average 7.1 hours) following a single 650 mg dose of aspirin. Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2576 Mechanism of Action Acetylsalicylic acid (ASA) blocks prostaglandin synthesis. It is non-selective for COX-1 and COX-2 enzymes. Inhibition of COX-1 results in the inhibition of platelet aggregation for about 7-10 days (average platelet lifespan). The acetyl group of acetylsalicylic acid binds with a serine residue of the cyclooxygenase-1 (COX-1) enzyme, leading to irreversible inhibition. This prevents the production of pain-causing prostaglandins. This process also stops the conversion of arachidonic acid to thromboxane A2 (TXA2), which is a potent inducer of platelet aggregation. Platelet aggregation can result in clots and harmful venous and arterial thromboembolism, leading to conditions such as pulmonary embolism and stroke. It is important to note that there is 60% homology between the protein structures of COX-1 and COX-2. ASA binds to serine 516 residue on the active site of COX-2 in the same fashion as its binding to the serine 530 residue located on the active site of COX-1. The active site of COX-2 is, however, slightly larger than the active site of COX-1, so that arachidonic acid (which later becomes prostaglandins) manages to bypass the aspirin molecule inactivating COX-2. ASA, therefore, exerts more action on the COX-1 receptor rather than on the COX-2 receptor. A higher dose of acetylsalicylic acid is required for COX-2 inhibition. Produce analgesia through a peripheral action by blocking pain impulse generation and via a central action, possibly in the hypothalamus. The peripheral action may predominate and probably involves inhibition of the synthesis or prostaglandins, and possibly inhibition of the synthesis and/or actions of other substances, which sensitize pain receptors to mechanical or chemical stimulation. /Salicylates/ Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2575 May produce antipyresis by acting centrally on the hypothalamic heat-regulating center to produce peripheral vasodilation resulting in increased cutaneous blood flow, sweating, and heat loss. The central action may involve inhibition of prostaglandin synthesis in the hypothalamus; however, there is some evidence that fevers caused by endogenous pyrogens that do not act via a prostaglandin mechanism may also respond to salicylate therapy. /Salicylates/ Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2575 |
| 分子式 |
C9H8O4
|
|
|---|---|---|
| 分子量 |
180.16
|
|
| 精确质量 |
180.042
|
|
| 元素分析 |
C, 60.00; H, 4.48; O, 35.52
|
|
| CAS号 |
50-78-2
|
|
| 相关CAS号 |
Aspirin;50-78-2; 50-78-2; 69-46-5 (calcium); 62952-06-1 (lysine); 23413-80-1 (Aspirin Aluminum); 552-98-7 (lithium); Deuterated Aspirin 921943-73-9; 97781-16-3
|
|
| PubChem CID |
2244
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
321.4±25.0 °C at 760 mmHg
|
|
| 熔点 |
134-136 °C(lit.)
|
|
| 闪点 |
131.2±16.7 °C
|
|
| 蒸汽压 |
0.0±0.7 mmHg at 25°C
|
|
| 折射率 |
1.551
|
|
| LogP |
1.19
|
|
| tPSA |
63.6
|
|
| InChi Key |
BSYNRYMUTXBXSQ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12)
|
|
| 化学名 |
2-acetyloxybenzoic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体外实验) |
配方 1 中的溶解度: ≥ 10 mg/mL (55.51 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 100.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 10 mg/mL (55.51 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 100.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 10 mg/mL (55.51 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 4% DMSO +PBS: 10mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.5506 mL | 27.7531 mL | 55.5062 mL | |
| 5 mM | 1.1101 mL | 5.5506 mL | 11.1012 mL | |
| 10 mM | 0.5551 mL | 2.7753 mL | 5.5506 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05932472 | Recruiting | Drug: Aspirin | Atherosclerosis Cardiovascular Diseases Drug Effect |
Tor Biering-Sørensen | January 15, 2024 | Phase 4 |
| NCT06228820 | January 15, 2024 | Drug: Aspirin 81Mg Ec Tab | Platelet Dysfunction Due to Drugs | The University of The West Indies | January 15, 2024 | Phase 2 |
| NCT04132791 | Terminated | Other: placebo after awakening + aspirin before bedtime Other: aspirin after awakening + placebo before bedtime |
Cardiovascular Diseases | Leiden University Medical Center | October 7, 2019 | Not Applicable |
| NCT03424408 | Completed | Drug: Aspirin 81 mg | Aspirin | Hamilton Health Sciences Corporation | March 1, 2018 | Not Applicable |
| NCT05604118 | Completed | Drug: Aspirin | Platelet Dysfunction Due to Aspirin | Cardiff University | August 1, 2016 | Not Applicable |