| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
阿斯巴甜由甲醇、天冬氨酸(中枢神经系统中的一种兴奋性神经递质)和苯丙氨酸(对神经递质调节至关重要)组成[2]。
|
|---|---|
| 体内研究 (In Vivo) |
在阿斯巴甜及其分解产物的急性、亚急性或长期毒性研究中,阿斯巴甜(4000 毫克/千克体重/天;口服)不会对小鼠、大鼠、仓鼠或狗造成任何负面影响[1]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorbed in the small intestine, aspartame is metabolized and absorbed very quickly. Metabolism / Metabolites Approximately 10% of aspartame (by weight) is broken down into methanol in the small intestine. Most of the methanol is absorbed and quickly converted into formaldehyde. Approximately 50% of aspartame (by weight) is broken down into phenylalanine. Approximately 40% of aspartame (by mass) is broken down into aspartic acid. Unlike some other intense sweeteners, aspartame is metabolized in the body and consequently has some nutritive value: 1 g provides approx 17 kJ (4 kcal). However, in practice, the small quantity of aspartame consumed provides a minimal nutritive effect. The use of aspartame has been of some concern owing to the formation of the potentially toxic metabolites methanol, aspartic acid, and phenylalanine. Of these materials, only phenylalanine is produced in sufficient quantities, at normal aspartame intake levels, to cause concern. Aspartame [SC-18862; 3-amino-N-(alpha-carboxyphenethyl) succinamic acid, methyl ester, the methyl ester of aspartylphenylalanine] is a sweetening agent that organoleptically has about 180 times the sweetness of sugar. The metabolism of aspartame has been studied in mice, rats, rabbits, dogs, monkeys, and humans. The compound was digested in all species in the same way as are natural constituents of the diet. Hydrolysis of the methyl group by intestinal esterases yielded methanol, which was oxidized in the one-carbon metabolic pool to CO2. The resultant dipeptide was split at the mucosal surface by dipeptidases and the free amino acids were absorbed. The aspartic acid moiety was transformed in large part to CO2 through its entry into the tricarboxylic acid cycle. Phenylalanine was primarily incorporated into body protein either unchanged or as its major metabolite, tyrosine. Although aspartame was hydrolyzed in the gut of the monkey to its constituent moieties, methanol, aspartic acid, and phenylalanine, the ingestion of 15 or 60 mg/kg doses for 10 days did not modify phenylalanine metabolism. Aspartame had little effect on the disappearance of iv admin (14)C-phenylalanine from the plasma, it did not substantially affect the conversion of phenylalanine into tyrosine or carbon dioxide, and it did not alter the rate of incorporation of label into protein. The majority of phenylalanine derived from aspartame was incorporated into body protein, with only 20-25% of the compound being excreted. 60-80% of the derived methanol and aspartic acid was oxidized to carbon dioxide. For more Metabolism/Metabolites (Complete) data for Aspartame (7 total), please visit the HSDB record page. Biological Half-Life At room temperature, aspartame is most stable at pH 4.3, where its half-life is nearly 300 days. At pH 7, its half-life is shortened to only a few days. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Aspartame is not detectable in breastmilk after maternal ingestion because it is rapidly broken down in the mother's body. An extremely large intake of aspartame (equivalent to 17 cans of soda or 100 packets of Equal Sweetener) can slightly increase the amount of phenylalanine in breastmilk. Phenylalanine concentrations in milk return to baseline by 12 hours after a large single dose of aspartame. Although it is prudent to avoid the use of aspartame in women who are nursing an infant with phenylketonuria, amounts that are typically ingested in aspartame-sweetened foods and beverages do not result in any additional risk to breastfed infants with phenylketonuria. Ingestion of diet drinks containing low-calorie sweeteners might increase the risk of vomiting in breastfed infants. An association between low-calorie sweeteners, and especially aspartame, and the risk of autism in boys has been found, but more data are needed to establish a cause-and-effect relationship. ◉ Effects in Breastfed Infants A cross-sectional survey assessed the dietary history of US mothers nursing infants between 11 and 15 weeks of age. The survey was used to estimate the amount of diet soda and fruit drinks consumed by the women. There were no statistically significant differences in infants’ weight or z-scores based on low calorie sweetener exposure. However, infants exposed to low calorie sweetener in milk once or less per week had a statistically significantly higher risk of vomiting than those who were not exposed. Greater exposure was not associated with vomiting. It was not possible to assess the effects of specific sweeteners. A retrospective dietary recall study compared the use of diet soda and aspartame during pregnancy and/or lactation to the risk of autism in the children. Among boys, autism was associated with three times the likelihood of exposure to aspartame. No statistically significant associations were found in girls. The contribution of exposure during breastfeeding was not separated from the risk of exposure during pregnancy, and intact aspartame is usually not found in milk, so breastmilk exposure cannot be claimed to cause autism based on these data. The authors propose that the methanol metabolite might have an impact on infants. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Interactions ... If mice are given aspartame in doses that elevate plasma phenylalanine levels more than those of tyrosine ... , the frequency of seizures following the administration of an epileptogenic drug, pentylenetetrazole, is enhanced. This effect is simulated by equimolar phenylalanine and blocked by concurrent administration of valine, which blocks phenylalanine's entry into the brain. Aspartame also potentiates the induction of seizures by inhaled fluorothyl or by electroconvulsive shock... Antimutagenic effects of combination of aspartame (0.4 and 4 mg/kg) and beta-carotene (0.15-15 mg/kg) were studied by estimation of chromosome aberrations in bone marrow cells of C57Bl/6 mice. Single and 5-day treatment with this combination decreased the clastogenic effects of dioxidine and cyclophosphamide and produced a more potent and universal antimutagenic effect than its constituents. The purpose of the present study was to investigate analgesic and anti-inflammatory properties of aspartame, an artificial sweetener and its combination with various opioids and NSAIDs for a possible synergistic response. The oral administration of aspartame (2-16 mg/kg, po) significantly increased the pain threshold against acetic acid-induced writhes in mice. Co-administration of aspartame (2mg/kg, po) with nimesulide (2 mg/kg, po) and naproxen (5 mg/kg, po) significantly reduced acetic acid-induced writhes as compared to effects per se of individual drugs. Similarly when morphine (1 mg/kg, po) or pentazocine (1 mg/kg, po) was co-administered with aspartame it reduced the number of writhes as compared to their effects per se. Aspartame (4,8,16 mg/kg, po) significantly decreased carrageenan-induced increase in paw volume and also reversed the hyperalgesic effects in rats in combination with nimesulide (2 mg/kg, po). The study indicated that aspartame exerted analgesic and anti-inflammatory effects on its own and have a synergistic analgesic response with conventional analgesics of opioid and non-opioid type, respectively. Ochratoxin A (OTA) is a mycotoxin produced by Aspergillus ochraceus as well as other molds. This mycotoxin contaminates animal feed and food. OTA is immunosuppressive, genotoxic, teratogenic, carcinogenic and is nephrotoxic in all animal species studied so far. OTA inhibits protein synthesis and induces lipid peroxidation. Since it seems impossible to avoid completely contamination of foodstuffs by toxigenic fungi, it is necessary to investigate the possible ways of limiting such toxicity. An attempt to prevent OTA-induced nephrotoxic and genotoxic effects, mainly the karyomegaly, has been made in vivo using aspartame (L-aspartyl-L-phenylalanine methyl ester), a structural analogue of both OTA and phenylalanine. Aspartame (25 mg/kg bw) prevented most of the nephrotoxic effects induced by OTA (289 ug/kg bw). It also showed some utility in preventing morphological and histological damage, mainly the karyomegaly. For more Interactions (Complete) data for Aspartame (9 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Aspartame is a dipeptide obtained by formal condensation of the alpha-carboxy group of L-aspartic acid with the amino group of methyl L-phenylalaninate. Commonly used as an artificial sweetener. It has a role as a sweetening agent, a nutraceutical, a micronutrient, a xenobiotic, an environmental contaminant, an apoptosis inhibitor and an EC 3.1.3.1 (alkaline phosphatase) inhibitor. It is a dipeptide, a carboxylic acid and a methyl ester. It is functionally related to a L-aspartic acid and a methyl L-phenylalaninate. It is a tautomer of an aspartame zwitterion.
Flavoring agent sweeter than sugar, metabolized as phenylalanine and aspartic acid. Flavoring agent sweeter than sugar, metabolized as PHENYLALANINE and ASPARTIC ACID. Drug Indication Used as a diet supplement and sugar substitute. Mechanism of Action 180 to 200 times sweeter than sucrose, it is metabolized as a protein and its subsequent amino-acids used up in there respective mechanisms. Therapeutic Uses Aspartame is used as an intense sweetening agent ... in pharmaceutical preparations including tablets, powder mixes, and vitamin preparations. It enhances flavor systems and can be used to mask some unpleasant taste characteristics; the approximate sweetening power is 80-200 times that of sucrose. Drug Warnings Aspartame is the methylester of a dipeptide composed of two amino acids, phenylalanine and aspartic acid. ... Persons with phenylketonuria, who must restrict carefully their phenylalanine intake, must be alerted to the presence of phenylalanine in the drug product and the amount of the ingredient in each dosage unit. Excessive use of aspartame should be avoided by patients with phenylketonuria. Aspartic acid and sodium glutamate were both neuroexcitatory amino acids which had an additive toxic effect on hypothalamic neurones. As this might be specially damaging to young children, who already receive sodium glutamate in gram quantities in their diet, aspartame should not generally be added to children's food. Reported adverse effects include: headaches; grand mal seizure; memory loss; gastrointestinal symptoms; and dermatological symptoms. However, scientifically controlled peer-reviewed studies have consistently failed to produce evidence of a causal effect between aspartame consumption and adverse health events ... For more Drug Warnings (Complete) data for Aspartame (8 total), please visit the HSDB record page. Pharmacodynamics Aspartame (L-alpha-aspartyl-L-phenylalanine methyl ester) is a low-calorie sweetener used to sweeten a wide variety of low- and reduced-calorie foods and beverages, including low-calorie tabletop sweeteners. Aspartame is composed of two amino acids, aspartic acid and phenylalanine, as the methyl ester. Aspartic acid and phenylalanine are also found naturally in protein containing foods, including meats, grains and dairy products. Methyl esters are also found naturally in many foods such as fruits and vegetable and their juices. Upon digestion, aspartame breaks down into three components (aspartic acid, phenylalanine and methanol), which are then absorbed into the blood and used in normal body processes. Neither aspartame nor its components accumulates in the body. These components are used in the body in the same ways as when they are derived from common foods. |
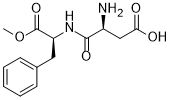
| 分子式 |
C14H18N2O5
|
|---|---|
| 分子量 |
294.3
|
| 精确质量 |
294.121
|
| CAS号 |
22839-47-0
|
| 相关CAS号 |
Aspartame-d5;1356849-17-6;Aspartame acesulfame;106372-55-8;Aspartame-d3;1356841-28-5
|
| PubChem CID |
134601
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
535.8±50.0 °C at 760 mmHg
|
| 熔点 |
242-248 °C
|
| 闪点 |
277.8±30.1 °C
|
| 蒸汽压 |
0.0±1.5 mmHg at 25°C
|
| 折射率 |
1.557
|
| LogP |
1.11
|
| tPSA |
118.72
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
21
|
| 分子复杂度/Complexity |
380
|
| 定义原子立体中心数目 |
2
|
| SMILES |
COC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@H](CC(=O)O)N
|
| InChi Key |
IAOZJIPTCAWIRG-QWRGUYRKSA-N
|
| InChi Code |
InChI=1S/C14H18N2O5/c1-21-14(20)11(7-9-5-3-2-4-6-9)16-13(19)10(15)8-12(17)18/h2-6,10-11H,7-8,15H2,1H3,(H,16,19)(H,17,18)/t10-,11-/m0/s1
|
| 化学名 |
(3S)-3-Amino-4-[[(2S)-1-methoxy-1-oxo-3-phenylpropan-2-yl]amino]-4-oxobutanoic acid
|
| 别名 |
Nutrasweet Asp-phe-ome AspartamAsp-Phe methyl ester
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~25 mg/mL (~84.95 mM)
H2O : ~5 mg/mL (~16.99 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.49 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.49 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (8.49 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 18.33 mg/mL (62.28 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3979 mL | 16.9895 mL | 33.9789 mL | |
| 5 mM | 0.6796 mL | 3.3979 mL | 6.7958 mL | |
| 10 mM | 0.3398 mL | 1.6989 mL | 3.3979 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02999321 | COMPLETED | Other: aspartame Other: water |
Oral Glucose Tolerance | Purdue University | 2016-08-17 | Not Applicable |
| NCT02520258 | COMPLETED | Other: Oral glucose tolerance test (OGTT) Other: Diet soda containing aspartame only |
Glucose Metabolism Disorder | Rockefeller University | 2015-08 | Not Applicable |
| NCT03232008 | UNKNOWN STATUS | Dietary Supplement: Canderel drink Dietary Supplement: Canderel+Lyle's Golden Syrup drink |
Appetitive Behavior Glucose Metabolism Disorders |
King's College London | 2015-09-01 | Not Applicable |
| NCT02569762 | COMPLETED | Dietary Supplement: Sucralose-Aspartame Dietary Supplement: Aspartame-Sucralose |
Impaired Glucose Tolerance | University of Manitoba | 2016-07 | Not Applicable |
| NCT05967741 | RECRUITING | Other: Erythritol Other: Aspartame |
Platelet Aggregation, Spontaneous Vascular Thrombosis | University of California, Davis | 2023-07-20 | Not Applicable |