| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| Other Sizes |
|

| 体外研究 (In Vitro) |
阿莫西林(阿莫西林)钠(1-100 µM;24 小时;嗜酸乳杆菌)以剂量依赖性方式减少活细胞并增加细胞壁破裂程度[1]。
|
|---|---|
| 体内研究 (In Vivo) |
当给予阿莫西林钠(7 mg/kg;ih;雌性ICR/瑞士小鼠)时,1 mg/L或更少的大鼠具有较高的存活率。它还可以抑制菌株数量。[2]
阿莫西林钠(1.6-9.5 mg/kg;口服;每天一次,持续 7 或 14 天;瑞士白化小鼠)可预防小鼠沙眼衣原体感染。 [3]。 |
| 动物实验 |
Animal Model: Female ICR/Swiss mice[2]
Dosage: 7 mg/kg Administration: Subcutaneous injection: every eight hours for a full day Result: exhibited a dose-dependent inhibition on the number of bacteria. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Limited information indicates that amoxicillin produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Occasionally, rash and disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush, have been reported, but these effects have not been adequately evaluated. Amoxicillin is acceptable in nursing mothers. Amoxicillin powder for suspension reconstituted with breastmilk is absorbed as well as the powder reconstituted with water. ◉ Effects in Breastfed Infants In a telephone follow-up study, 25 nursing mothers reported taking amoxicillin (dosage unspecified). Three mothers reported diarrhea in their infants. No rashes or candidiasis were reported among the exposed infants. In contrast, a small, controlled, prospective study had mothers monitor their infants for signs of adverse effects (furring of the tongue, feeding difficulties, changes in stool frequency and consistency, diaper rash, and skin rash). Weight change and the development of jaundice were also recorded. No statistical differences in these parameters were found between the infants of the control mothers and those of mothers taking the related antibiotics, ampicillin or ampicillin-clavulanate. A prospective, controlled study asked mothers who called an information service about adverse reactions experienced by their breastfed infants. Of 40 infants exposed to amoxicillin in breastmilk, 2 developed diarrhea and 1 developed a rash. A study compared the breastfed infants of mothers taking amoxicillin to those taking a macrolide antibiotic. Adverse reactions occurred in 8.3% of the infants exposed to amoxicillin, which was similar to the rate in macrolide-exposed infants. Reactions included rash and somnolence. A 2-month-old infant breastfed since birth. His mother had taken many medications during pregnancy, but she did not recall their identity. She developed mastitis and was treated with amoxicillin/clavulanic acid 1 gram orally every 12 hours and gentamicin 160 mg intramuscularly once daily. The infant was breastfed for 10 minutes starting 15 minutes after the first dose of both drugs. About 20 minutes later, the infant developed a generalized urticaria which disappeared after 30 minutes. A few hours later, the infant breastfed again and the urticaria reappeared after 15 minutes and disappeared after an hour. After switching to formula feeding and no further infant exposure to penicillins, the reaction did not reappear with follow-up to 16 months of age. The adverse reaction was probably caused by the antibiotics in breastmilk. The drug that caused the reaction cannot be determined, but it was most likely the amoxicillin/clavulanic acid. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| 参考文献 | |
| 其他信息 |
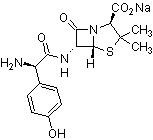
Amoxicillin sodium is an organic sodium salt that is the monosodium salt of amoxicillin. It contains an amoxicillin(1-).
Amoxicillin Sodium is the sodium salt form of a broad-spectrum, semisynthetic aminopenicillin antibiotic with bactericidal activity. Amoxicillin binds to and inactivates penicillin-binding proteins (PBPs) located on the inner membrane of the bacterial cell wall. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. This interrupts bacterial cell wall synthesis and results in the weakening of the bacterial cell wall and causes cell lysis. A broad-spectrum semisynthetic antibiotic similar to AMPICILLIN except that its resistance to gastric acid permits higher serum levels with oral administration. |
| 分子式 |
C16H18N3O5S.NA
|
|---|---|
| 分子量 |
387.39
|
| 精确质量 |
387.086
|
| 元素分析 |
C, 49.61; H, 4.68; N, 10.85; Na, 5.93; O, 20.65; S, 8.28
|
| CAS号 |
34642-77-8
|
| 相关CAS号 |
Amoxicillin;26787-78-0;Amoxicillin trihydrate;61336-70-7;Amoxicillin-d4;2673270-36-3;Amoxicillin trihydrate mixture with potassium clavulanate (4:1);Amoxicillin arginine;59261-05-1
|
| PubChem CID |
23663126
|
| 外观&性状 |
White to light yellow solid powder
|
| 沸点 |
743.2ºC at 760 mmHg
|
| tPSA |
161.09
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
596
|
| 定义原子立体中心数目 |
4
|
| SMILES |
S1C(C([H])([H])[H])(C([H])([H])[H])[C@]([H])(C(=O)[O-])N2C([C@@]([H])([C@@]12[H])N([H])C(C([H])(C1C([H])=C([H])C(=C([H])C=1[H])O[H])N([H])[H])=O)=O.[Na+]
|
| InChi Key |
BYHDFCISJXIVBV-GJUCOGTPSA-M
|
| InChi Code |
InChI=1S/C16H19N3O5S.Na/c1-16(2)11(15(23)24)19-13(22)10(14(19)25-16)18-12(21)9(17)7-3-5-8(20)6-4-7;/h3-6,9-11,14,20H,17H2,1-2H3,(H,18,21)(H,23,24);/q;+1/p-1/t9?,10-,11+,14-;/m1./s1
|
| 化学名 |
sodium (2S,5R,6R)-6-(2-amino-2-(4-hydroxyphenyl)acetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
|
| 别名 |
Amoxicillin sodium; Lamoxy; Penamox; BRL-2333AB-B; Moxacin;
|
| HS Tariff Code |
2934.99.03.00
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 78~100 mg/mL ( 201.34~258.14 mM )
Water : 78~100 mg/mL(~258.14 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.37 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1 mg/mL (2.58 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 10.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1 mg/mL (2.58 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.08 mg/mL (5.37 mM) 配方 5 中的溶解度: 100 mg/mL (258.14 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5814 mL | 12.9069 mL | 25.8138 mL | |
| 5 mM | 0.5163 mL | 2.5814 mL | 5.1628 mL | |
| 10 mM | 0.2581 mL | 1.2907 mL | 2.5814 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。