| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The toxicokinetics of PABA is characterized by fast oral absorption, biotransformation by the major routes acetylation and glycine conjugation, the minor route by glucuronidation in the liver and kidney, and a fast and almost complete elimination via the urine within 24 hrs. PABA is extensively acetylated during percutaneous absorption in humans. Studies have shown that PABA can cross the placenta rapidly. Furthermore, the results of one study indicate that the human placenta has a significant capacity for acetylation of PABA. Of an oral dose of 1 g para-aminobenzoic acid, 82% was excreted in the urine of 3 male volunteers within 4 hr; para-aminohippuric acid and acetyl-para- aminohippuric acid were the principal metabolites. Concurrent administration of sodium benzoate totally abolished the excretion of these glycine conjugates. The percutaneous absorption and metabolism of three structurally related compounds, benzoic acid, p-aminobenzoic acid (PABA), and ethyl aminobenzoate (benzocaine), were determined in vitro through hairless guinea pig skin. Benzocaine was also studied in human skin. Absorption of benzocaine was rapid and similar through both viable and nonviable skin. The absorption of the two acidic compounds, benzoic acid and PABA, was greater through nonviable skin. A small portion (6.9%) of absorbed benzoic acid was conjugated with glycine to form hippuric acid. Although N-acetyl-benzocaine had not been observed as a metabolite of benzocaine when studied by other routes of administration, both PABA and benzocaine were extensively N-acetylated during percutaneous absorption. Thus, the metabolism of these compounds should be considered in an accurate assessment of absorption after topical application. Skin absorption of PABA corresponding to 1.6 to 9.6% of the applied amount of PABA was measured in the urine of six male volunteers after application of PABA in three different preparations. No significant difference where observed between the three preparations. For more Absorption, Distribution and Excretion (Complete) data for 4-AMINOBENZOIC ACID (10 total), please visit the HSDB record page. Metabolism / Metabolites The two major metabolic pathways in a variety of species (guinea-pigs, rabbits and rats, but not dogs) are acetylation of the amino group and conjugation of the carboxy group, either with glycine or with glucuronic acid. Acetylation occurs in liver, heart, lung, blood and kidneys of rats and in the mucous membranes of the gastrointestinal tract of cattle. N-Acetyl-transferase activity in the presence of para-aminobenzoic acid is similar in liver and lung tissue of rabbits. Acetylation not only of para-aminobenzoic acid (30-40%) but also of para-aminohippuric acid (70%) takes place in the kidney of rabbits; acetylation of para-aminohippurate also occurs in the kidney of guinea-pigs. Acetylation is dose-dependent. In rats given up to 5 mg/kg bw, 75% of the metabolites were acetylated; with higher doses, the extent of acetylation decreased down to 40%. An inverse relationship exists between acetylation and glycine conjugation: when acetylation decreases, glycine conjugation increases; such a decrease is seen in pantothenic acid-deficient rats. . Male rats excreted a larger amount of acetylated conjugates in the urine than females. Following ingestion /in man/ of 1.0 g, ...main metabolites were p-aminohippuric acid and acetyl-p-aminohippuric acid. ... PABA is predominantly metabolized by acetylation and glycine conjugation to form p-acetamidobenzoic acid (PAABA), p-aminohippuric acid (PAHA), and p-acetamidohippuric acid (PAAHA). ... The half-lives of PABA were 7.01 +/- 0.32 min in rapid acetylation rabbits and 7.08 +/- 0.78 min in slow acetylation rabbits. Significant differences were obtained in formation of PAABA and PAHA formed from PABA in both acetylation phenotype rabbits. For more Metabolism/Metabolites (Complete) data for 4-AMINOBENZOIC ACID (9 total), please visit the HSDB record page. Biological Half-Life ... The half-lives of PABA were 7.01 +/- 0.32 min in rapid acetylation rabbits and 7.08+/-0.78 min in slow acetylation rabbits. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
Sulfonamides act by competitive inhibition of PABA in the microorganism. PABA administration in sufficient doses thus antagonized the antibacterial effect of the sulfonamides. PABA appears to block the formatin of salicyluric acid from salicylic acid, resulting in increased salicylate blood levels. Aminosalicylic acid appears to act on tubercle bacilli in a manner similar to that of sulfonamides on other organisms (by competing with PABA). Thus the administration of PABA may be expected to inhibit the antimicrobial activity of aminosalicylic acid. Interferon titers induced by 0.007% PABA and poludan were compatible after both injections in the conjunctiva but not in the anterior chamber humor. For more Interactions (Complete) data for 4-AMINOBENZOIC ACID (14 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rabbit iv 2000 mg/kg LD50 Rabbit oral 1830 mg/kg LD50 Dog oral 1000 mg/kg LD50 Mouse oral 2850 mg/kg LD50 Rat oral 6,000 mg/kg bw |
| 其他信息 |
Therapeutic Uses
Sunscreening Agent Used orally in the treatment of conditions such as scleroderma, dermatomyositis, and Peyronie's disease, and topically as a sunscreen and protectant. Daily use of a sunscreen with a high SPF (greater than 15) on usually exposed skin is recommended for residents of areas of high ... /solar radiation/ who work outdoors or ... /enjoy/ regular outdoor recreation. Daily use of a sunscreen can reduce the cumulative ... /solar/ exposure that causes actinic keratoses and squamous-cell carcinoma. PABA has long been an accepted objective marker to verify completeness of 24 hour urine sampling as PABA is rapidly and almost completely eliminated with the urine. For this reason PABA has been used clinically for long as the indicator substance in pancreas and liver function tests. Sunscreen preparations should be applied uniformly and generously to all exposed skin surfaces, including lips, before exposure to UVB radiation. Two applications of the sunscreen may be needed for maximum protection. PABA-containing sunscreens are most effective when applied 1-2 hours before exposure to sunlight. Sunscreen products that are not water resistant should be reapplied after swimming, towel-drying, or profuse sweating and, because most sunscreens are easily removed from the skin, reapplication every 1-2 hours or according to the manufacturer's directions usually is required to provide adequate protection from UVB light. /Sunscreens/ Drug Warnings PABA has been shown in vitro to displace methotrexate from plasma protein binding, thus increasing the free methotrexate concentrations. PABA derivatives reportedly have weak sensitization potential, but the incidence of allergic and photoallergic contact dermatitis associated with their use is increasing. Contamination of PABA derivatives with benzocaine which may cause allergic reactions has been reported. In patients allergic to compounds that are structurally similar to PABA (e.g., ester-type anesthetics, aniline dyes, thiazides, sulfonylurea and paraphenylenediamine drugs), cross-sensitivity to PABA derivatives has been reported occasionally; therefore, sunscreens containing PABA derivatives may be contraindicated in patients with a history of hypersensitivity to these chemicals. The manufacturers of sunscreen preparations with propellants warn that concentrating and subsequently inhaling the fumes from these preparations may be harmful or fatal. /Propellants/ Because the absorptive characteristics of skin of children younger than 6 months of age may differ from those of adults and because the immaturity of metabolic and excretory pathways of these children may limit their ability to eliminate any percutaneously absorbed sunscreen agent, sunscreen products should be used in children younger than 6 months of age only as directed by a clinician. It is possible that the characteristics of geriatric skin also differ from those of skin in younger adults, but these characteristics and the need for special considerations regarding use of sunscreen preparations in this age group are poorly understood. /Sunscreens/ For more Drug Warnings (Complete) data for 4-AMINOBENZOIC ACID (13 total), please visit the HSDB record page. |
| 分子式 |
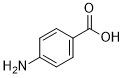
C7H7NO2
|
|---|---|
| 分子量 |
137.1360
|
| 精确质量 |
137.047
|
| CAS号 |
150-13-0
|
| 相关CAS号 |
25136-77-0
|
| PubChem CID |
978
|
| 外观&性状 |
Off-white to light brown solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
339.9±25.0 °C at 760 mmHg
|
| 熔点 |
187-189 °C(lit.)
|
| 闪点 |
159.4±23.2 °C
|
| 蒸汽压 |
0.0±0.8 mmHg at 25°C
|
| 折射率 |
1.637
|
| LogP |
0.83
|
| tPSA |
63.32
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
10
|
| 分子复杂度/Complexity |
128
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
ALYNCZNDIQEVRV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C7H7NO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4H,8H2,(H,9,10)
|
| 化学名 |
4-aminobenzoic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~75 mg/mL (~546.89 mM)
H2O : ~4.55 mg/mL (~33.18 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (18.23 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (18.23 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (18.23 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 4.17 mg/mL (30.41 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶 (<60°C). 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.2918 mL | 36.4591 mL | 72.9182 mL | |
| 5 mM | 1.4584 mL | 7.2918 mL | 14.5836 mL | |
| 10 mM | 0.7292 mL | 3.6459 mL | 7.2918 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。