| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
Endogenous Metabolite; Microbial Metabolite
|
|---|---|
| 体外研究 (In Vitro) |
尿囊素以其抗炎和抗氧化特性而闻名,是化妆品中的常见成分[1]。尿囊素以剂量依赖性方式提高 STZ 产生的 β 细胞的活力,同时减弱细胞毒性和细胞凋亡。尿囊素增强磷酸化 B 细胞淋巴瘤 2 (Bcl-2) 的表达并降低 caspase-3。咪唑啉 3 (I3) 受体已被证明可被尿囊素激活 [2]。
|
| 体内研究 (In Vivo) |
尿囊素亚慢性治疗(1、3 或 10 mg/kg,持续 7 天)显着增加东莨菪碱诱导的胆碱能阻断和潜伏期,在正常幼鼠的被动回避任务中进行评估。尿囊素治疗(3 或 10 mg/kg,持续 7 天)还升高了磷酸化磷脂酰肌醇 3-激酶 (PI3K)、磷酸化蛋白激酶 B (Akt) 和磷酸化糖原合成酶激酶 3β (GSK-3β) 的表达水平。尿囊素可显着增强海马齿状回区域未成熟神经元的神经细胞增殖[1]。对 STZ 治疗的大鼠每天注射尿囊素 8 天,可显着降低血浆葡萄糖并提高血浆胰岛素水平 [2]。尿囊素在 30 分钟时降低 SHR 血压,这是最有效的时间。此外,用尿囊素治疗的 SHR 以剂量依赖性方式显示出抗高血压作用。此外,在镇静大鼠中,尿囊素可降低心肌收缩力和心率。此外,尿囊素可以显着改善外周血流量[3]。
|
| 酶活实验 |
ApoTox-Glo三重测定法[2]
将β细胞以每孔1×104个细胞的总密度接种到96孔板中。每个孔包含200µl RPMI 1640培养基和适当的测试化合物。根据制造商的说明使用ApoTox-Glo Triplex测定法来测量β细胞的活力、细胞毒性和凋亡。24小时后,将含有GF-AFC底物和双-AAF-R110底物的活力/细胞毒性试剂加入所有孔中并孵育30分钟。将Caspase-Glo 3/7加入孔中并短暂混合30 s,然后在室温下孵育30 min。在380EX/510EM处测量荧光以评估生存能力,在485EX/520EM处测量细胞毒性,并测量发光以评估细胞凋亡。 |
| 细胞实验 |
将原代培养的细胞分成6孔板。除去培养基,并用磷酸盐缓冲盐水(PBS)洗涤细胞一次。将含有25mM葡萄糖的RPMI 1640培养基与5mM STZ一起加入到每个孔中,并孵育6小时以诱导细胞凋亡。为了了解尿囊素在保护胰腺β细胞抵抗STZ中的作用,在添加5mM STZ前30分钟提供不同剂量的尿囊素预处理,并孵育6小时。为了鉴定尿囊素的信号通路,如前所述,在添加尿囊素前30分钟提供1µM KU14R:I3结合位点拮抗剂,或1µM U73122:磷脂酶C(PLC)抑制剂。去除所有培养基,并在处理前用PBS洗涤细胞三次,以评估形态[2]。
|
| 动物实验 |
Mice: For memory ameliorating study, mice are administered vehicle solution, allantoin (1, 3 or 10 mg/kg, p.o.) or donepezil (5 mg/kg, p.o.) at the same time (10:00-12:00 a.m) and same place for 7 days. For memory enhancing study, mice are administered vehicle solution, allantoin (1, 3 or 10 mg/kg, p.o.) or piracetam (200 mg/kg, i.p.). The final administration of allantoin, donepezil or piracetam is performed 1 h before an acquisition trial in the passive avoidance task[1]
Glucose and insulin levels in STZ-treated rats: The induction of pancreatic cell damage was accomplished by injecting 45 mg/kg STZ dissolved in 10 mM Na-citrated buffer intraperitoneally. STZ-treated rats with blood glucose above 200 mg/dl at 7 days post-injection were included in the group. Total of 24 rats were divided into three groups as follows: Control (STZ) (n = 8), STZ + allantoin (n = 8), STZ + KU14R + allantoin (n = 8). The third group was treated with an intravenous injection of 8 mg/kg/day KU14R; the first and second groups were treated with the same volume of vehicle injected intravenously. After 30 min of KU14R injection, the second and third groups received 10 mg/kg/day of allantoin intravenously. The first group was injected the same volume of vehicle intravenously. The experiments were performed for 8 days. The blood samples were obtained from tail vein everyday. The plasma glucose levels were measured everyday, and the plasma insulin levels were measured on day 0, 4, 6, 8 [2]. Rats: Animals are randomly divided into four groups: (I) the control group treated with the vehicle, saline; (II) the allantoin group treated by intravenous injection of allantoin at 0.5 mg/kg; (III) the allantoin+efaroxan group treated with allantoin at the most effective dose (0.5 mg/kg, i.v.) and efaroxan at effective dose (1.5 mg/kg, i.v.) 30 minutes before injection of allantoin; and (IV) the allantoin treated SHRs group treated by intravenous injection of allantoin at various dose for desired time. After treatment of allantoin, the rats are placed into a holder for the determination of the mean blood pressure[3]. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
In studies on human subjects, a recovery of 19% and 34% of allantoin in the urine was observed but only in two individuals and only after the administration of massive doses of allantoin. After intravenous administration, recovery in the urine was practically quantitative with doses of 75 to 600 mgm in the human model. After 240 mgm, excretion continued for 72 hours in human subjects and the results were similar in regards to subcutaneous injection. Urinary clearance is the predominant excretion route. Some studies suggest that the average renal clearance of allantoin in normal, healthy human subjects is approximately 123 cc per minute. It is generally agreed upon that exogenously administered allantoin is rapidly excreted. Allantoin administered to dogs orally as solid or solution was excreted in the urine to an extent of between 35 and 92 per cent within 24 hours. No allantoin was recovered either in urine or feces when given to rabbits orally. In man the recovery was 19 and 34 per cent in two individuals after massive doses. After intravenous administration recovery in the urine was practically quantitative with doses of 75 to 600 mgm. in the dog and in man. After 240 mgm. in man excretion continued for 72 hours. The results were similar after subcutaneous injection. Uric acid injected intravenously into a dog was converted into allantoin within two hours. Metabolism / Metabolites Uricase is the enzyme that possesses the functionality to convert uric acid to allantoin. Considering humans do not possess any endogenous uricase, uric acid is the only final breakdown product in the purine degradation of unwanted waste product purine nucleotides. The presence of allantoin in human urine is subsequently the result of non-enzymatic processes on uric acid with reactive oxygen species. Such non-enzymatic processes are consequently potentially suitable biomarkers for measuring oxidative stress in chronic illnesses and aging. Furthermore, as allantoin is found endogenously and is part of basic, natural metabolic pathways, no accumulation is expected of it. Additionally, allantoin is not believed to be metabolized to a measurable extent in humans and animals. In humans, uric acid is the final breakdown product of unwanted purine nucleotides. Uric acid is the last stage in purine degradation, because humans lack the enzyme uricase which converts uric acid into allantoin. Allantoin in the presence of calcium ions has been implicated as a potential toxic agent in Reye's syndrome. An investigation of possible alternative sources of allantoin in humans, which lack the enzyme uricase, has been initiated. Urate is a strong reducing agent which can reduce cytochrome c nonenzymatically, with the concomitant production of CO2 and H+. The stoichiometries measured for the various reactants and products were 1 urate:2 cytochrome c:1 H+:1 CO2. The initial reaction rate depended on the concentrations of both urate and cytochrome c, with reaction kinetics that were first order with respect to urate and second order with respect to cytochrome c. The participation of molecular oxygen in this reaction could not be detected. The pH and ionic strength optima for this reaction were determined to be 9.5-10.5 and 10(-5) M, respectively. Based on the results reported here, the following balanced equation can be written: urate-2 + 2 cytochrome c+3 + 2 H2O----allantoin + 2 cytochrome c+2 + H+ + HCO3-. /The authors/ propose that allantoin can be generated from the oxidation of urate by cytochrome c+3, and that this is a potential source of allantoin in human tissues. Uric acid is the main nitrogenous waste product in birds but it is also known to be a potent antioxidant. Hominoid primates and birds lack the enzyme urate oxidase, which oxidizes uric acid to allantoin. Consequently, the presence of allantoin in their plasma results from non-enzymatic oxidation. In most mammals purine degradation ultimately leads to the formation of allantoin. Humans lack the enzyme uricase, which catalyzes the conversion of uric acid to allantoin. For more Metabolism/Metabolites (Complete) data for ALLANTOIN (11 total), please visit the HSDB record page. Biological Half-Life When studied in cattle, sheep, and horses, the half-life of allantoin is in the range of 1 to 2.5 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
Feed containing 0.2% allantoin ... with or without 0.2% sodium nitrite, was given ad lib. to groups of 20 or 24 male and 20 or 24 female F344 rats for 106 wk. ... Control rats were given untreated feed ... and nitrite-treated controls were given sodium nitrite at a concentration of 0.2% in feed or drinking-water. At the end of the treatment period the rats were given untreated feed ...and observed until death. There was little or no life-shortening effect in any treatment group. /Allantoin / administered alone /and in combination with sodium nitrite did not induce/ an increase in the incidence of any tumor in comparison with the untreated control groups ... |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
A urea hydantoin that is found in URINE and PLANTS and is used in dermatological preparations. Allantoin, a component in Comfrey, stimulates tissue repair and wound healing through cell proliferation. Allantoin has also had significant effect on cellular multiplication in degenerating and regenerating peripheral nerves. In humans, the allantoin to uric acid ratio in plasma increases during oxidative stress, thus this ratio has been suggested to be an in vivo marker for oxidative stress in humans. Diagnostic marker for oxidative stress during antituberculous (anti-TB) therapy. For more Therapeutic Uses (Complete) data for ALLANTOIN (8 total), please visit the HSDB record page. Drug Warnings Skin: For external use only. Ocular: Avoid contact with eyes. Sensitization: Mederma is contraindicated in individuals who have shown hypersensitivity to any of its components /Mederma/ Pharmacodynamics There is no well controlled and appropriate data that can formally substantiate the pharmacodynamic properties of allantoin. Nevertheless, ongoing studies suggest that allantoin possesses moisturizing and keratolytic effects, as well as abilities to increase the water content of the extracellular matrix and enhance the desquamation of upper layers of dead skin cells, all of which are activities that can promote cell proliferation and facilitate wound healing. |
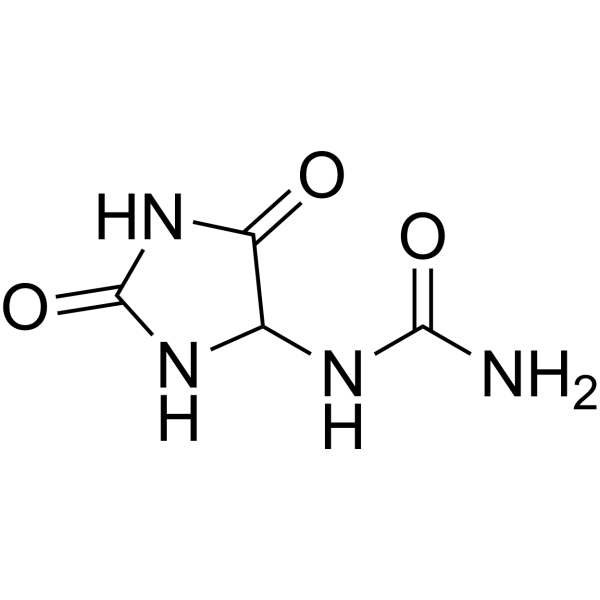
| 分子式 |
C4H6N4O3
|
|---|---|
| 分子量 |
158.1154
|
| 精确质量 |
158.043
|
| 元素分析 |
C, 30.39; H, 3.83; N, 35.43; O, 30.36
|
| CAS号 |
97-59-6
|
| 相关CAS号 |
Allantoin-13C2,15N4;1219402-51-3; 97-59-6 (racemic); 7303-80-2 (R-isomer); 3844-67-5 (S-isomer)
|
| PubChem CID |
204
|
| 外观&性状 |
White to off-white solid
|
| 密度 |
1.7±0.1 g/cm3
|
| 沸点 |
478ºC
|
| 熔点 |
230 °C (dec.)(lit.)
|
| 闪点 |
230-234°C
|
| 折射率 |
1.616
|
| 来源 |
Microbe
|
| LogP |
-2.89
|
| tPSA |
113.32
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
11
|
| 分子复杂度/Complexity |
225
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C(NC1C(=O)NC(=O)N1)N
|
| InChi Key |
POJWUDADGALRAB-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C4H6N4O3/c5-3(10)6-1-2(9)8-4(11)7-1/h1H,(H3,5,6,10)(H2,7,8,9,11)
|
| 化学名 |
1-(2,5-dioxoimidazolidin-4-yl)urea
|
| 别名 |
5-Ureidohydantoin; SD 101; Allantion; Sebical; Septalan; Allantol; Cordianine; NSC 7606; DL-Allantoin; Glyoxyldiureid; Glyoxyldiureide; Glyoxylic diureide; Psoralon;
|
| HS Tariff Code |
2933.21.0000
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~316.22 mM)
H2O : ~3.85 mg/mL (~24.35 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (15.81 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (15.81 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (15.81 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 2 mg/mL (12.65 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶 (<60°C). 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.3243 mL | 31.6216 mL | 63.2431 mL | |
| 5 mM | 1.2649 mL | 6.3243 mL | 12.6486 mL | |
| 10 mM | 0.6324 mL | 3.1622 mL | 6.3243 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05796635 | Completed | Other: Herpecin L | Cold Sores | Focus Consumer Healthcare | 2023-01-04 | Not Applicable |
| NCT04046783 | Completed | Device: patch | Cesarean Section; Dehiscence Scar Keloid Wound Heal |
University of Salerno | 2019-03-02 | |
| NCT05105139 | Completed | Other: Allantoin/ Coal Tar/ Clioquinol
Other: Allantoin/ Coal Tar/ Clioquinol/ Triclosan |
Psoriasis of Scalp Seborrheic Dermatitis |
Laboratorios Silanes S.A. de C.V. | 2021-11-29 | |
| NCT00825565 | Completed | Drug: Alwextin cream | Epidermolysis Bullosa | Northwestern University | 2009-02 | Phase 2 |
| NCT01863407 | Unknown status | Drug: DAM Drug: Normal Saline |
Postoperative Ileus | Beijing Bozhiyin T&S Co., Ltd. | 2013-04 | Phase 3 |
|
|
|