| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|

| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Approximately 90% of orally administered glucosamine (salt form) gets absorbed from the small intestine. Metabolism / Metabolites A significant fraction of ingested glucosamine is catabolized by first-pass metabolism in the liver. |
|---|---|
| 参考文献 | |
| 其他信息 |
Aldehydo-N-acetyl-D-glucosamine is the open-chain form of N-acetyl-D-glucosamine. It has a role as a human metabolite.
The N-acetyl derivative of glucosamine. N-Acetylglucosamine is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). D-Glucose, 2-(acetylamino)-2-deoxy- has been reported in Arabidopsis thaliana, Homo sapiens, and other organisms with data available. The N-acetyl derivative of glucosamine. See also: Poliglusam (monomer of); Adomiparin (monomer of); Adomiparin Sodium (monomer of) ... View More ... Drug Indication For the treatment and prevention of osteoarthritis, by itself or in combination with chondroitin sulfate. Mechanism of Action The mechanism of action in relieving arthritic pain and in repair of cartilage is a matter of speculation. Biochemically, glucosamine is involved in glycoprotein metabolism. Glycoproteins, known as proteoglycans, form the ground substance in the extra-cellular matrix of connective tissue. Proteoglycans are polyanionic substances of high-molecular weight and contain many different types of heteropolysaccharide side-chains covalently linked to a polypeptide-chain backbone. These polysaccharides make up to 95% of the proteoglycan structure. In fact, chemically, proteoglycans resemble polysaccharides more than they do proteins. The polysaccharide groups in proteoglycans are called glycosaminoglycans (GAGs). GAGs include hyaluronic acid, chondroitin sulfate, dermatan sulfate, keratan sulfate, heparin and heparan sulfate. All of the GAGs contain derivatives of glucosamine or galactosamine. Glucosamine derivatives are found in hyaluronic acid, keratan sulfate and heparan sulfate. Chondroitin sulfate contains derivatives of galactosamine. The glucosamine-containing glycosaminoglycan hyaluronic acid is vital for the function of articular cartilage. GAG chains are fundamental components of aggrecan found in articular cartilage. Aggrecan confers upon articular cartilage shock-absorbing properties. It does this by providing cartilage with a swelling pressure that is restrained by the tensile forces of collagen fibers. This balance confers upon articular cartilage the deformable resilience vital to its function. In the early stages of degenerative joint disease, aggrecan biosynthesis is increased. However, in later stages, aggrecan synthesis is decreased, leading eventually to the loss of cartilage resiliency and to most of the symptoms that accompany osteoarthritis. During the progression of osteoarthritis, exogenous glucosamine may have a beneficial role. It is known that, in vitro, chondrocytes do synthesize more aggregan when the culture medium is supplemented with glucosamine. N-acetylglucosamine is found to be less effective in these in vitro studies. Glucosamine has also been found to have antioxidant activity and to be beneficial in animal models of experimental arthritis. The counter anion of the glucosamine salt (i.e. chloride or sulfate) is unlikely to play any role in the action or pharmacokinetics of glucosamine. Further, the sulfate in glucosamine sulfate supplements should not be confused with the glucosamine sulfate found in such GAGs as keratan sulfate and heparan sulfate. In the case of the supplement, sulfate is the anion of the salt. In the case of the above GAGs, sulfate is present as an ester. Also, there is no glucosamine sulfate in chondroitin sulfate (source: PDRhealth). |
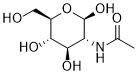
| 分子式 |
C8H15NO6
|
|---|---|
| 分子量 |
221.209
|
| 精确质量 |
221.089
|
| CAS号 |
7512-17-6
|
| 相关CAS号 |
D-N-Acetylgalactosamine;1811-31-0;N-Acetyl-D-glucosamine-13C;253679-94-6;N-Acetyl-D-glucosamine-13C2,15N;478529-44-1;N-Acetyl-D-glucosamine-13C,15N-1;N-Acetyl-D-glucosamine-13C6;1194446-34-8;N-Acetyl-D-glucosamine-13C-1;478518-87-5;N-Acetyl-D-glucosamine-13C-2;478518-89-7;N-Acetyl-D-glucosamine-18O;N-Acetyl-D-glucosamine-13C-3;478529-39-4;N-Acetyl-D-glucosamine-15N;478518-85-3;N-Acetyl-D-glucosamine-13C,15N;478529-40-7;N-Acetyl-D-glucosamine-13C3,15N;478529-43-0
|
| PubChem CID |
1738118
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
595.4±50.0 °C at 760 mmHg
|
| 熔点 |
201-204ºC
|
| 闪点 |
313.9±30.1 °C
|
| 蒸汽压 |
0.0±3.8 mmHg at 25°C
|
| 折射率 |
1.576
|
| LogP |
-2.48
|
| tPSA |
127.09
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
15
|
| 分子复杂度/Complexity |
221
|
| 定义原子立体中心数目 |
4
|
| SMILES |
CC(=O)N[C@@H](C=O)[C@H]([C@@H]([C@@H](CO)O)O)O
|
| InChi Key |
OVRNDRQMDRJTHS-FMDGEEDCSA-N
|
| InChi Code |
InChI=1S/C8H15NO6/c1-3(11)9-5-7(13)6(12)4(2-10)15-8(5)14/h4-8,10,12-14H,2H2,1H3,(H,9,11)/t4-,5-,6-,7-,8-/m1/s1
|
| 化学名 |
N-Acetyl-beta-D-glucosamine
|
| 别名 |
Acetylglucosamine GlcNAc Bio-NAG N-Acetylglucosamine N-Acetyl-D-glucosamineNSC 400525 NSC 524344GreenNAG Marine Sweet
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~125 mg/mL (~565.07 mM)
H2O : ~100 mg/mL (~452.06 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 3.25 mg/mL (14.69 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 32.5 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 3.25 mg/mL (14.69 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 32.5 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 3.25 mg/mL (14.69 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 50 mg/mL (226.03 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.5206 mL | 22.6030 mL | 45.2059 mL | |
| 5 mM | 0.9041 mL | 4.5206 mL | 9.0412 mL | |
| 10 mM | 0.4521 mL | 2.2603 mL | 4.5206 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04706416 | Completed Has Results | Dietary Supplement: N-acetyl glucosamine (NAG) |
Coronavirus Covid19 |
Quantinosis.ai LLC | November 14, 2020 | Phase 1 |
| NCT06268483 | Completed | Dietary Supplement: cranberry, D-mannose, propolis extract, tumeric and Boswellia |
Urinary Tract Infections, Recurrent | Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico |
March 1, 2022 | Not Applicable |
| NCT02511041 | Terminated | Drug: N-Acetylglucosamine (GlcNAc) Drug: Uridine |
PGM3 | National Institute of Allergy and Infectious Diseases (NIAID) |
June 30, 2015 | Phase 1 |
| NCT02196909 | Completed | Other: motor function and strength assessment |
HIBM | Institut de Myologie, France | July 2014 | Not Applicable |