| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
SphK2 ( IC50 = 60 μM )
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:ABC294640 显着改变神经酰胺/S1P 的比例,这与 MDA-MB-231 细胞中 SK 活性的抑制一致。 ABC294640 抑制肿瘤细胞增殖,IC50 值范围约为 6 至 48 μM,并削弱肿瘤细胞迁移并伴随微丝损失。 ABC294640 可诱导 A-498、PC-3 和 MDA-MB-231 细胞中的非凋亡细胞死亡、溶酶体形态变化、自噬体形成以及酸性囊泡增加。在 MCF-7 和 ER 转染的 HEK293 细胞中,ABC294640 降低 E2 刺激的 ERE 荧光素酶活性。激酶测定:ABC294640 和 DMS 的 IC50 值通过新开发的基于 HPLC 的 SK 活性测定测定。简而言之,将测试化合物与重组 SK1 或 SK2 和 NBD-Sph 在下文详述的同工酶选择性测定缓冲液中与 1 mg/ml 无脂肪酸牛血清白蛋白、100 μM ATP 和 400 μM MgCl2 一起孵育。产物,即 NBD-S1P,通过 HPLC 与 NBD-Sph 分离,如下: Waters 2795 HPLC 系统,配有 Waters 2495 荧光检测器、C8 Chroolith RP-8e 色谱柱 (100 × 4.6 mm)、1 ml/min 流动相(乙腈/20 mM 磷酸钠缓冲液,pH2.5,45:55)。通过 465 nm 激发和 531 nm 发射监测荧光。 NBD-S1P/(NBD-Sph + NBD-S1P) 的比率用作 SK 活性的量度。 SK-同工酶选择性检测缓冲液各自含有 20 mM Tris、pH7.4、5 mM EDTA、5 mM EGTA、3 mM β-巯基乙醇、5% 甘油、1× 蛋白酶抑制剂和 1× 磷酸酶抑制剂。对于 SK1 测定缓冲液,添加 0.25%(最终)Triton X-100;对于 SK2 缓冲液,添加 1 M(最终)KCl。测定在室温下进行 2 小时,然后添加 1.5 倍体积的甲醇以终止激酶反应。 10 分钟后,将样品在 20,000g 下离心以沉淀沉淀的蛋白质,并通过 HPLC 分析上清液。在确定 ABC294640 对 SK2 抑制的 Ki 的实验中,ADP Quest 测定系统用于测量不同浓度鞘氨醇和 ABC294640 存在下的激酶活性。为了确定 ABC294640 对细胞 SK 活性的影响,将接近汇合的 MDA-MB-231 细胞进行血清饥饿过夜,然后用不同浓度的 ABC294640 处理。然后将细胞与终浓度为 1 μM 的 [3H] 鞘氨醇一起孵育。细胞吸收外源鞘氨醇,通过 SK 活性将其转化为 S1P,通过提取将 [3H]S1P 与 [3H] 鞘氨醇分离,并通过闪烁计数进行定量。细胞测定:为了确定测试化合物对增殖的影响,细胞(1025LU、Hep-G2、A-498、MCF-7、Caco-2、MDA-MB-231、HT-29、Panc-1、DU145、 T24 和 SK-OV-3 细胞系)接种到 96 孔微量滴定板中并贴壁 24 小时。将不同浓度的 ABC294640 添加到各个孔中,并将细胞再孵育 72 小时。在此期间结束时,使用磺基罗丹明结合测定法测定活细胞的数量。杀死细胞的百分比计算为与对照培养物相比磺基罗丹明结合的减少百分比。使用 GraphPad Prism 进行抑制曲线的回归分析。
|
||
| 体内研究 (In Vivo) |
Opaganib (ABC294640)·HCl的抗肿瘤活性在同基因肿瘤模型中进行了测试,该模型使用小鼠JC乳腺腺癌细胞系在免疫能力强的BALB/c小鼠皮下生长(Lee et al., 2003)。由于上述良好的口服吸收,我们确定口服给药ABC294640·HCl在体内降低肿瘤生长的能力。该SK抑制剂在奇数天以3.5至100mg /kg的剂量给药于禁食小鼠。每天监测体重和肿瘤体积。如图7所示,ABC294640·HCl引起乳腺腺癌异种移植物生长的剂量依赖性降低。在研究过程中,每个治疗组的体重与给药小鼠相比保持不变(数据未显示)。将ABC294640·HCl在肿瘤研究中的药效与上述毒性数据进行比较,发现其治疗指数大于7 (250 mg/kg无毒剂量/ 35 mg/kg抗肿瘤活性)。因此,该SK2抑制剂具有良好的治疗指数。[1]
为了确定Opaganib (ABC294640)·HCl的抗肿瘤作用是由该化合物介导的,我们采用LC/MS定量其在肿瘤中的积累。在这些实验中,轴承JC肿瘤异种移植小鼠服用100毫克/公斤ABC294640·由腹腔内注射盐酸的浓度测量复合等离子体和肿瘤2和5 h。图8中所示,大约75μg / ml(197μM) ABC294640出现在等离子体2 h,这减少了56个μg / ml(147μM) 5 h。大量的ABC294640在肿瘤2和5 h测定36和54μg / g湿重,分别分别约为94 μM和140 μM(假设1g约等于1ml)。因此,在完整小鼠的肿瘤中,ABC294640的含量远高于阻断细胞增殖所需的含量[1]。 每天给药50mg /kg Opaganib (ABC294640)在4周的治疗中导致肿瘤生长延迟具有统计学意义。28天后,切除肿瘤组织,固定,切片,免疫染色,评估beclin 1和LC3的水平,TUNEL染色,确定凋亡细胞的数量(图6B)。如图6C所示,暴露于ABC294640小鼠的肿瘤中,beclin 1和LC3的染色强度均高于对照小鼠。[2] 在携带乳腺癌异种移植物的小鼠中,ABC294640(100 mg/kg,口服)可显着减少肿瘤生长,这与 S1P 水平的消耗有关。在携带 A-498 异种移植物的严重联合免疫缺陷小鼠中,ABC294640 延迟了肿瘤生长并提高了自噬标记物。 ABC294640 可防止肝移植引起的炎症以及先天性免疫和适应性免疫之间的串扰、诱发和加剧移植物损伤的重大事件,并改善肝功能和存活率。 |
||
| 酶活实验 |
最近开发的基于 HPLC 的 SK 活性测定用于确定 ABC294640 和 DMS 的 IC50 值。简而言之,将测试化合物与重组 SK1 或 SK2 和 NBD-Sph 在下述同工酶选择性测定缓冲液中孵育,该缓冲液含有 400 μM MgCl2、100 μM ATP 和 1 mg/ml 不含脂肪酸的牛血清白蛋白。以下是 HPLC 如何将产品或 NBD-S1P 与 NBD-Sph 分离: 使用 Waters 2495 荧光检测器、C8 Chromolith RP-8e 色谱柱 (100 × 4.6 mm) 和 1 ml/min 流动相 (pH 2.5) Waters 2795 HPLC 系统由磷酸钠缓冲液与乙腈/20 mM 45:55 组成。在 465 nm 激发波长和 531 nm 发射波长下观察到荧光。 NBD-S1P/(NBD-Sph + NBD-S1P) 比率用于计算 SK 活性水平。每种 SK-同工酶选择性测定缓冲液中均含有 20 mM Tris、pH7.4、5 mM EDTA、5 mM EGTA、3 mM β-巯基乙醇、5% 甘油、1× 蛋白酶抑制剂和 1× 磷酸酶抑制剂。将 0.25%(最终)Triton X-100 添加到 SK1 测定缓冲液中,并将 1 M(最终)KCl 添加到 SK2 缓冲液中。测定在室温下运行两小时后,通过添加 1.5 倍体积的甲醇来终止激酶反应。将样品在 20,000 g 下离心 10 分钟以除去沉淀的蛋白质,然后将上清液进行 HPLC 分析。 ADP Quest 测定系统用于在实验中测量不同浓度的鞘氨醇和 ABC294640 存在下的激酶活性,以确定 ABC294640 抑制 SK2 的 Ki。为了确定 ABC294640 对细胞 SK 活性的影响,接近汇合的 MDA-MB-231 细胞经历过夜血清饥饿方案,然后暴露于不同浓度的 ABC294640。接下来,将[3H]鞘氨醇以1μM的终浓度添加到细胞中。细胞吸收外源鞘氨醇,通过 SK 活性将其转化为 S1P,通过提取将 [3H]S1P 与 [3H] 鞘氨醇分离,并通过闪烁计数进行定量。
|
||
| 细胞实验 |
细胞周期、细胞凋亡和线粒体膜的完整性分析。[2]
为了进行细胞周期分析,将细胞暴露于不同浓度Opaganib (ABC294640)中24、48或72小时,用PBS洗涤两次,并在0.5 ml PI染色溶液(50 μg/ml碘化丙啶,40 μg/ml RNase A在PBS中)中37℃孵育30分钟。使用Becton Dickinson FACSCalibur流式细胞仪在南卡罗来纳医科大学流式细胞仪设备中分析细胞周期分布。根据制造商的说明,用caspase-Glo 3/7法测定caspase 3和caspase 7的活性。简而言之,a -498细胞生长在白色96孔板中,每孔密度为10,000个细胞。Opaganib (ABC294640)孵育后,加入100 μl caspase试剂,室温孵育30 min。孵育后,使用SpectraMax M5读板仪测定发光水平。暴露于顺铂的细胞作为细胞凋亡的阳性对照。对于Annexin- v染色,在暴露于Opaganib (ABC294640)后,将细胞胰蛋白酶化,在含10%胎牛血清的培养基中重悬,在PBS中洗涤两次,并在Annexin结合缓冲液(10 mM HEPES, 140 mM NaCl和2.5 mM CaCl2, pH 7.4)中重悬。在细胞悬液100 μl的基础上,加入5 μl Annexin- v溶液,室温保存15 min。孵育后,加入400 μl Annexin缓冲液,立即流式细胞术分析细胞。为了分析线粒体膜功能,细胞暴露于的Opaganib (ABC294640)或顺铂(阳性对照),在生长培养基中用100 nM四甲基罗丹明染色30分钟,PBS洗涤后,立即流式细胞术分析细胞。收集贴壁细胞和漂浮细胞进行细胞凋亡和流式细胞术分析。 为了确定测试化合物对增殖的影响,将细胞(1025LU、Hep-G2、A-498、MCF-7、Caco-2、MDA-MB-231、HT-29)接种到 96 孔微量滴定板中。 、Panc-1、DU145、T24 和 SK-OV-3 细胞系)并使其粘附一整天。单独的孔中充满不同浓度的 Opaganib (ABC294640),并将细胞再孵育 72 小时。使用磺基罗丹明结合测定,在此时确定活细胞的数量。作为与对照培养物相比磺基罗丹明结合的百分比,计算被杀死的细胞的百分比。 GraphPad Prism 用于执行抑制曲线的回归分析。 |
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption of ABC294640.[1]
The HCl salt of ABC294640 (ABC294640·HCl) has been synthesized in multigram amounts for characterizations of its toxicity, pharmacokinetics, and in vivo efficacy. Formulation analyses were conducted to identify a suitable pharmaceutical composition for in vivo studies. We chose five different oral formulations from the Division of Drug Information Resources' Inactive Ingredient Guide, a compendium of all inactive ingredients in approved drug products currently marketed for human use, to assess their ability to support oral absorption of ABC294640. Solutions of ABC294640·HCl in water, 90% propylene glycol, 100% polyethylene glycol 400 (PEG400), 50% PEG400 or 0.375% Polysorbate-80 did not precipitate (measured as turbidity at 590 nm), and so were administered to fasted female Swiss-Webster mice at a dose of 100 mg/kg. Blood samples were removed at 1 and 7 h, and plasma levels of ABC294640 were determined by use of an internal standard and reverse-phase HPLC coupled to an ion-trap quadrupole mass spectrometer running in positive SIM detection mode. As shown in Table 3, substantial amounts of ABC294640 were detected in the blood 1 h after oral dosing, with the highest levels attained in the samples formulated in 90% propylene glycol. It should be noted that these ABC294640 concentrations are sufficient to inhibit SK activity and proliferation of tumor cells. By 7 h, the plasma concentrations decreased by approximately 50% in most cases. Effective absorption was observed in the sample formulated in 0.375% Polysorbate-80, and this solvent for ABC294640·HCl was used in further pharmacokinetic and efficacy analyses because of its low toxicity. [1] To further understand the absorption properties of ABC294640·HCl, the relationship between plasma concentration and dose was examined. Mice were orally dosed with 10, 35, or 100 mg/kg ABC294640, and the plasma levels were determined at 30 min. As shown in Fig. 5, the plasma ABC294640 values demonstrated a good linear response with doses up to at least 100 mg/kg. Pharmacokinetics of ABC294640.[1] Detailed pharmacokinetic studies were performed on ABC294640·HCl in 0.375% Polysorbate-80. Female Swiss-Webster mice were dosed with 50 mg/kg ABC294640 either intravenously or orally. Groups of mice (3 per group) were anesthetized, and blood was removed via cardiac puncture at time points ranging from 1 min to 7 h. Plasma concentrations of ABC294640 were determined by LC/MS, and pharmacokinetic parameters were calculated by use of the WinNolin software package (Table 4). Intravenous administration of ABC294640 resulted in high plasma concentrations that were eliminated with a half-time of clearance of 1.4 h. Although the peak plasma level of ABC294640 was lower when the compound was administered by oral gavage, the compound was much more persistent, probably reflecting continued absorption from the gastrointestinal tract, such that the calculated half-time for clearance was 4.5 h. It is noteworthy that comparison of the oral versus the intravenous pharmacokinetics of ABC294640 indicated an excellent oral bioavailability of 66% (F = AUCoral/AUCiv). |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity of ABC294640.[1]
Preliminary toxicity studies were performed to determine the appropriate dose for in vivo efficacy testing. No immediate or delayed toxicity was observed in female Swiss-Webster mice treated with intraperitoneal doses of ABC294640·HCl up to at least 250 mg/kg. Repeated injections in the same mice every other day over 15 days showed a similar lack of toxicity at doses up to at least 250 mg/kg. Dose-escalation toxicity testing was performed via oral gavage with ABC294640·HCl dissolved in 0.375% Polysorbate-80, and no toxic effects were observed after administration of doses up to 1000 mg/kg. Therefore, the compound was considered to be suitable for more detailed in vivo studies. Non-good laboratory practice acute toxicology studies were contracted to Eurofins|Product Safety Laboratories, in which ABC294640·HCl was given orally in 0.375% Polysorbate-80 to rats at doses of 0, 100, or 250 mg/kg daily for 7 days. There were no clinical observations or gross findings that were considered to be the result of ABC294640·HCl administration or otherwise. There were no significant changes in total body weight of the treated animals, although there was a slight decrease in body weight gain, consistent with a small decrease in food consumption, in the high-dose rats. Hematology studies (Table 5) indicated decreases in red blood cell number and hematocrit of approximately 20% in animals given either 100 or 250 mg/kg/day; and a slight increase in neutrophils and decrease in basophils in the treated rats. These changes would be scored as grade 0 toxicities on the standard National Cancer Institute scale for evaluating toxicity in clinical trials. It is noteworthy that no decreases in lymphocyte, platelet, or granulocyte counts were observed, indicating that the compound does not induce immunologic toxicities that are common with other anticancer drugs. Likewise, there were no drug-induced alterations of a broad panel of clinical chemistry or coagulation parameters. No gross abnormalities were noted for any of the euthanized animals when necropsied at the end of the 7-day observation period. Likewise, there were no treatment-related microscopic changes in any organ examined in the high-dose group, except for a slight decrease in the background level of extramedullary hematopoiesis in the spleen that may underlie the small decreases in the hematocrit. To further characterize the hematologic changes observed in the acute study, mice were treated with 0, 100, or 250 mg of ABC294640·HCl/kg daily for 28 days. As indicated in Fig. 6A, mice treated with 250 mg/kg experienced a 20% decrease in red blood cell count and hematocrit, and a modest increase in the number of circulating neutrophils on day 7, essentially identical to the previous study with rats. However, after 28 days of treatment (Fig. 6B), these parameters were restored to normal levels, indicating that the animals had fully recovered from any transient impairment of hematopoiesis. In addition, there were no changes in the brain or spleen weights of treated mice, whereas a slight decrease (12%) in liver weight was observed in mice treated with 250 mg/kg. |
||
| 参考文献 | |||
| 其他信息 |
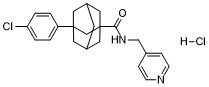
3-(4-chlorophenyl)-N-(pyridin-4-ylmethyl)-1-adamantanecarboxamide is an organochlorine compound.
Opaganib, also known as ABC294640, is a selective [sphingosine kinase-2 (SK2)](https://go.drugbank.com/polypeptides/Q9NRA0) inhibitor that is orally administered. This drug has potential anticancer, anti-inflammatory, and antiviral activities, with potential applications in oncology, inflammation, the gastrointestinal system, and COVID-19. Opaganib is an orally available, aryladamantane compound and selective inhibitor of sphingosine kinase-2 (SK2) with potential antineoplastic activity. Upon administration, opaganib competitively binds to and inhibits SK2, thereby preventing the phosphorylation of the pro-apoptotic amino alcohol sphingosine to sphingosine 1-phosphate (S1P), the lipid mediator that is pro-survival and critical for immunomodulation. This may eventually lead to the induction of apoptosis and may result in an inhibition of cell proliferation in cancer cells overexpressing SK2. SK2 and its isoenzyme SK1 are overexpressed in numerous cancer cell types. Mechanism of Action Opaganib selectively inhibits [sphingosine kinase-2 (SK2)](https://go.drugbank.com/polypeptides/Q9NRA0). This inhibition blocks the synthesis of sphingosine 1-phosphate (S1P) and its activities (which includes regulation of fundamental biological processes such as cell proliferation, migration, immune cell trafficking, angiogenesis, immune-modulation, and suppression of innate immune responses from T-cells). This drug has dual anti-inflammatory and antiviral activity targeting a host cell component and is unaffected by viral mutation, contributing to minimization of the likelihood of resistance. It is currently being investigated against COVID-19 as it has demonstrated anti-SARS-CoV-2 activity in studies. Sphingolipid-metabolizing enzymes control the dynamic balance of the cellular levels of important bioactive lipids, including the apoptotic compound ceramide and the proliferative compound sphingosine 1-phosphate (S1P). Many growth factors and inflammatory cytokines promote the cleavage of sphingomyelin and ceramide leading to rapid elevation of S1P levels through the action of sphingosine kinases (SK1 and SK2). SK1 and SK2 are overexpressed in a variety of human cancers, making these enzymes potential molecular targets for cancer therapy. We have identified an aryladamantane compound, termed ABC294640 [3-(4-chlorophenyl)-adamantane-1-carboxylic acid (pyridin-4-ylmethyl)amide], that selectively inhibits SK2 activity in vitro, acting as a competitive inhibitor with respect to sphingosine with a K(i) of 9.8 muM, and attenuates S1P formation in intact cells. In tissue culture, ABC294640 suppresses the proliferation of a broad panel of tumor cell lines, and inhibits tumor cell migration concomitant with loss of microfilaments. In vivo, ABC294640 has excellent oral bioavailability, and demonstrates a plasma clearance half-time of 4.5 h in mice. Acute and chronic toxicology studies indicate that ABC294640 induces a transient minor decrease in the hematocrit of rats and mice; however, this normalizes by 28 days of treatment. No other changes in hematology parameters, or gross or microscopic tissue pathology, result from treatment with ABC294640. Oral administration of ABC294640 to mice bearing mammary adenocarcinoma xenografts results in dose-dependent antitumor activity associated with depletion of S1P levels in the tumors and progressive tumor cell apoptosis. Therefore, this newly developed SK2 inhibitor provides an orally available drug candidate for the treatment of cancer and other diseases.[1] The sphingolipids ceramide, sphingosine, and sphingosine 1-phosphate (S1P) regulate cell signaling, proliferation, apoptosis, and autophagy. Sphingosine kinase-1 and -2 (SK1 and SK2) phosphorylate sphingosine to form S1P, shifting the balanced activity of these lipids toward cell proliferation. We have previously reported that pharmacological inhibition of SK activity delays tumor growth in vivo. The present studies demonstrate that the SK2-selective inhibitor 3-(4-chlorophenyl)-adamantane-1-carboxylic acid (pyridin-4-ylmethyl)amide (ABC294640) induces nonapoptotic cell death that is preceded by microtubule-associated protein light chain 3 cleavage, morphological changes in lysosomes, formation of autophagosomes, and increases in acidic vesicles in A-498 kidney carcinoma cells. ABC294640 caused similar autophagic responses in PC-3 prostate and MDA-MB-231 breast adenocarcinoma cells. Simultaneous exposure of A-498 cells to ABC294640 and 3-methyladenine, an inhibitor of autophagy, switched the mechanism of toxicity to apoptosis, but decreased the potency of the SK2 inhibitor, indicating that autophagy is a major mechanism for tumor cell killing by this compound. Induction of the unfolded protein response by the proteasome inhibitor N-(benzyloxycarbonyl)leucinylleucinylleucinal Z-Leu-Leu-Leu-al (MG-132) or the heat shock protein 90 inhibitor geldanamycin synergistically increased the cytotoxicity of ABC294640 in vitro. In severe combined immunodeficient mice bearing A-498 xenografts, daily administration of ABC294640 delayed tumor growth and elevated autophagy markers, but did not increase terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling-positive cells in the tumors. These data suggest that ABC294640 promotes tumor cell autophagy, which ultimately results in nonapoptotic cell death and a delay of tumor growth in vivo. Consequently, ABC294640 may effectively complement anticancer drugs that induce tumor cell apoptosis.[2] |
| 分子式 |
C23H26CL2N2O
|
|---|---|
| 分子量 |
417.37
|
| 精确质量 |
416.142
|
| 元素分析 |
C, 66.19; H, 6.28; Cl, 16.99; N, 6.71; O, 3.83
|
| CAS号 |
1185157-59-8
|
| 相关CAS号 |
1185157-59-8 (HCl);915385-81-8;
|
| PubChem CID |
71587942
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| LogP |
6.531
|
| tPSA |
45.48
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
551
|
| 定义原子立体中心数目 |
1
|
| SMILES |
ClC1C=CC(=CC=1)C12C[C@@H]3CC(CC(C(NCC4C=CN=CC=4)=O)(C3)C1)C2.Cl
|
| InChi Key |
BXGLNXNRKRUYTH-ZKOGBWAVSA-N
|
| InChi Code |
InChI=1S/C23H25ClN2O.ClH/c24-20-3-1-19(2-4-20)22-10-17-9-18(11-22)13-23(12-17,15-22)21(27)26-14-16-5-7-25-8-6-16;/h1-8,17-18H,9-15H2,(H,26,27);1H/t17-,18?,22?,23?;/m0./s1
|
| 化学名 |
3-(4-chlorophenyl)-N-(pyridin-4-ylmethyl)adamantane-1-carboxamide hydrochloride
|
| 别名 |
Opaganib; ABC294640 hydrochloride; UNII-RFN6U0F30B; ABC-294640 HCl; RFN6U0F30B; 1185157-59-8; Tricyclo(3.3.1.13,7)decane-1-carboxamide, 3-(4-chlorophenyl)-N-(4-pyridinylmethyl)-, hydrochloride (1:1); (7S)-3-(4-chlorophenyl)-N-(pyridin-4-ylmethyl)adamantane-1-carboxamide;hydrochloride; ABC-294640; ABC294640; ABC 294640; ABC294640 HCl
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3960 mL | 11.9798 mL | 23.9596 mL | |
| 5 mM | 0.4792 mL | 2.3960 mL | 4.7919 mL | |
| 10 mM | 0.2396 mL | 1.1980 mL | 2.3960 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04207255 | Active Recruiting |
Drug: Opaganib Drug: Abiraterone |
Prostate Cancer | Medical University of South Carolina |
March 27, 2020 | Phase 2 |
| NCT04414618 | Completed | Drug: Opaganib Drug: Placebo |
Coronavirus Infections | RedHill Biopharma Limited | July 2, 2020 | Phase 2 |
| NCT04435106 | Completed | Drug: Opaganib Drug: Standard of Care |
Coronavirus Infections | Shaare Zedek Medical Center | April 3, 2020 | N/A |
| NCT04467840 | Completed | Drug: Opaganib Drug: Placebo |
COVID-19 Lung Infection |
RedHill Biopharma Limited | August 21, 2020 | Phase 2 Phase 3 |
| NCT03377179 | Completed | Drug: ABC294640 Drug: Hydroxychloroquine Sulfate 200 MG |
Cholangiocarcinoma Cholangiocarcinoma, Perihilar |
RedHill Biopharma Limited | March 7, 2018 | Phase 2 |