| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Oral administration. The absorption, distribution, and elimination of pyraclostrobin were studied in male and female Wistar rats (aged at least 7 weeks) after oral administration of pyraclostrobin (purity, >98%) radiolabelled with carbon-14 at either the tolyl or chlorophenyl rings. ... In a series of four experiments, the excretion of pyraclostrobin was studied in excreta collected at 6, 12 and 24 hr after dosing, and at 24 hr intervals thereafter for 168 hr, or until 90% of the applied radioactivity had been excreted. In the first three experiments, groups of four male and four female rats were given a single oral dose of 14C-tolyl- or 14C-chlorophenyl-labelled pyraclostrobin or unlabelled pyraclostrobin at 50 mg/kg bw. In the fourth experiment, four rats of each sex were given a single oral dose of 14C-tolyl-labelled pyraclostrobin at 5 mg/kg bw. At the end of each of these experiments, the animals were sacrificed and the heart, liver, spleen, bone, skin, lung, ovaries, bone marrow, carcass, muscle, kidney, testes, brain, pancreas, uterus, adipose tissue, stomach and contents, thyroid glands, adrenal glands, blood/plasma and intestinal tract and contents were assessed for radioactivity. Exhaled air was also collected from two males in each of the two experiments using radiolabelled pyraclostrobin in order to determine exhalation of 14C-labelled gases. Two additional experiments were conducted to examine blood concentrations of radioactivity after administration of 14C-tolyl-labelled pyraclostrobin at 5 or 50 mg/kg bw. Blood samples (100-200 uL) were taken from animals at 0.5, 1, 2, 4, 8, 24, 48, 72, 96 and 120 hr after dosing, and the amount of radioactivity in whole blood and plasma was assessed. Tissue distribution was examined in animals sacrificed at 0.5, 8, 20 and 42 hr after dosing at 5 mg/kg bw, and at 0.5, 24, 36 and 72 hr after dosing at 50 mg/kg bw. The heart, liver, spleen, bone, skin, lung, ovaries, bone marrow, carcass, muscle, kidney, testes, brain, pancreas, uterus, adipose tissue, stomach and contents, thyroid glands, adrenal glands, blood/plasma and intestinal tract and contents were assessed for radioactivity. To examine biliary excretion of pyraclostrobin, bile ducts of the animals were cannulated and bile was collected at 3 hr intervals until 48 hr after administration of 14C-tolyl-labelled pyraclostrobin at 5 or 50 mg/kg bw in four animals of each sex at each dose (the duration depended on the health of the animals and the excretion rate at later time-points). In rats given a single dose of 14C-tolyl-labelled pyraclostrobin at either 5 or 50 mg/kg bw, plasma concentrations of radioactivity initially peaked after 0.5 to 1 hr; there was a secondary peak after 8 hr in males at 5 or 50 mg/kg bw and females given 5 mg/kg bw, and after 24 hR in females given 50 mg/kg bw. The magnitude of the difference in the time to peak for females, given the high dose, is likely to be at least partially artifactual owing to the absence of a sampling point between 8 and 24 hr. After the second peak, plasma concentrations declined to <0.1 ug equivalent/g after 120 hr. The terminal half-lives were similar in males and females, but were 50% longer at 5 mg/kg bw than at 50 mg/kg bw. The area under the curve of plasma concentration-time was approximately proportional to dose for each sex, indicating that absorption was not saturated at the higher dose. After a single oral dose of 14C-tolyl-labelled pyraclostrobin at 50 mg/kg bw, the highest concentrations of radioactivity /in rats/ were found in the gastrointestinal tract (gut, 28 to 39 ug equivalent/g; gut contents, 63 to 92 ug equivalent/g; stomach, 325 to 613 ug equivalent/g; stomach contents, 1273 to 1696 ug equivalent/g) after 0.5 hr. The liver (13 to 25 ug equivalent/g) had higher concentrations of radioactivity than the kidneys (4 to 7 ug equivalent/g) and plasma (2 to 6 ug equivalent/g), with lowest values being recorded in the bone (0.1 to 0.3 ug equivalent/g) and brain (1 to 2 ug equivalent/g). After 72 hr, tissues and organs contained <2.6 ug equivalent/g. After a dose of 5 mg/kg bw, the highest concentrations of radioactivity were also found in the gastrointestinal tract (gut, 5 ug equivalent/g; gut contents, 7 to 9 ug equivalent/g; stomach, 49 to 89 ug equivalent/g; stomach contents, 160 to 205 ug equivalent/g) after 0.5 hr. After 42 hr, tissues and organs contained <0.7 ug equivalent/g. In rats that were pretreated with unlabelled pyraclostrobin for 14 days and given a single oral dose of 14C-tolyl-labelled pyraclostrobin at 5 mg/kg bw, the highest concentrations of radioactivity after 120 hr were found in the thyroid gland (0.18 to 0.35 ug equivalent/g) and the liver (0.1 ug equivalent/g). In all other tissues, the concentration of radioactivity recorded was <0.1 ug equivalent/g. The rapid and essentially complete excretion of pyraclostrobin and the decline of tissue concentrations to low levels over the observation period, suggests a low potential for accumulation. The overall recovery of radioactivity was 91 to 105% in all /four oral experiments in rats/. In the first 48 hr after a single oral dose of 14C-tolyl-labelled pyraclostrobin at 5 or 50 mg/kg bw, 10 to 13% of the administered radioactivity was excreted in the urine and 74 to 91% was excreted in the feces. The total amount of radioactivity excreted in the urine and feces after 120 hr was 11 to 15% and 81 to 92%, respectively. A similar pattern of excretion was observed in rats that were pre-treated with unlabelled pyraclostrobin for 14 days and given a single oral dose of 14C-tolyl-labelled pyraclostrobin at 5 mg/kg bw of (12 to 13% in the urine and 76 to 77% in the feces after 48 hr; 12 to 14% in the urine and 79 to 81% in the feces after 120 hr) and in rats given a single oral dose of chlorophenyl-labelled pyraclostrobin at 50 mg/kg bw (11 to 15% in the urine and 68 to 85% in the feces after 48 hr; 12 to 16% in the urine and 74 to 89% in the feces after 120 hr). There was no detectable radioactivity in the expired air from rats treated with 14C-tolyl- or 14C-chlorophenyl-labelled pyraclostrobin at 50 mg/kg bw. In tissues and organs, the radioactivity that remained after 120 hr was <1 mg equivalent/g at 50 mg/kg bw and <0.1 mg equivalent/g at 5 mg/kg bw. Within 48 hr after administration of 14C-tolyl-labelled pyraclostrobin at 5 or 50 mg/kg bw of, 35 to 38% of the administered radioactivity was excreted via the bile, indicating, in conjunction with observations on urinary excretion, that approximately 50% of the administered dose had been absorbed. Dermal application. The absorption and, to a limited extent, the distribution and excretion of 14C-labelled pyraclostrobin (in Solvesso) in groups of 16 male Wistar rats was assessed after a single dermal application at a nominal dose of 0.015, 0.075 or 0.375 mg/cm2, corresponding to 0.15, 0.75 and 3.75 mg/animal or approximately 0.8, 4 and 18 mg/kg bw. Animals were exposed to the test material for 4 (four rats per group) or 8 (12 rats per group) hr and four rats per group were sacrificed at 4, 8, 24 or 72 hr after the start of the exposure. An area of approximately 10 cm2 on the shoulders was clipped free of hair and was washed with acetone 24 hr before dosing. A silicone ring was glued to the skin and the test substance preparation (10 uL/cm2) was administered with a syringe, which was weighed before and after application. A nylon mesh was then glued to the surface of the silicone ring and covered with a porous bandage. After the exposure period, the protective covers were removed and the exposed skin was washed with a soap solution. After sacrifice, the concentration of radioactivity in the excreta, blood cells, plasma, liver, kidneys, carcass, treated and untreated skin was assessed. Radioactivity in the cage and skin wash and the protective covering, including the silicone ring, was also assessed. In all groups, 99 to 110% of the radioactivity was recovered. At sacrifice at 72 hr, after an 8 hr exposure, 1.6 to 2.6% of the administered dose was absorbed, 22 to 26% was on the skin or in the skin wash, and 72 to 80% was recovered on the protective cover. Only 0.2 to 0.4% and 0.9 to1.8% was excreted in the urine and faeces, respectively. For more Absorption, Distribution and Excretion (Complete) data for PYRACLOSTROBIN (6 total), please visit the HSDB record page. Metabolism / Metabolites Tissues, excreta and bile from animals used in the toxicokinetics studies and from additional groups given a single dose at 50 mg/kg bw per day (to provide more material for analysis) were analysed for metabolites of pyraclostrobin. In order to determine the metabolites in the plasma, liver and kidneys, additional groups were treated with a single dose of 14C-tolyl- or 14C-chlorophenol ring-labelled pyraclostrobin at 5 and 50 mg/kg bw and sacrificed 8 hr later. Metabolites were identified using high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS) and nuclear magnetic resonance (NMR). The metabolism of pyraclostrobin proceeded through three main pathways primarily involving alterations to the three major portions of the pyraclostrobin molecule. The methoxy group on the tolyl-methoxycarbamate moiety was readily lost, with few major metabolites retaining this group. Hydroxylation of the aromatic and/or pyrazole rings was followed by glucuronide and occasionally sulfate conjugation, and many metabolites were derived from the chlorophenol-pyrazole or tolyl-methoxycarbamate moieties of pyraclostrobin, following cleavage of the ether linkage, with subsequent ring hydroxylation and glucuronide or sulfate conjugation. Metabolites were similar in both sexes and at all doses. No unchanged parent compound was found in the bile or urine and only small amounts in the faeces. Compounds dominating the identified metabolites recovered from the urine were: ring-hydroxylated pyraclostrobin; the chlorophenol pyrazole moiety hydroxylated on the pyrazole ring with or without a sulfate conjugate; a glucuronide of the tolyl-methoxycarbamate moiety; and a benzoic acid derivative of the tolyl-methoxycarbamate moiety. In the feces, the dominant metabolite was a demethoxylated and pyrazole ring hydroxylated pyraclostrobin. In the bile, the primary metabolite was a glucuronide of pyraclostrobin hydroxylated on the pyrazole ring at the 4' position and this compound, together with the demethoxylated derivative found in the faeces, was also the dominant metabolite isolated from the plasma and the liver. Demethoxylation of the methoxycarbamate moiety appeared to occur primarily in the gut, as the major metabolite in the bile retains this group intact whereas in the feces the major metabolite is the demethoxylated derivative. Most of the radiolabel isolated from the kidneys was in the form of the unchanged parent compound and a demethoxylated derivative. Wistar rats were dosed ... with chlorophenyl-labeled pyraclostrobin (>98% chemical purity, >98% radiochemical purity) or tolyl-labeled pyraclostrobin (>98% chemical purity, >98% radiochemical purity), adjusted with unlabeled pyraclostrobin (BAS 500 F), 99.8 % purity to desired dose. ... Tissue samples were collected 8 hr after dosing, to achieve maximal tissue levels for analysis. Data did not demonstrate sex differences. Dose levels (5 or 50 mg/kg) and treatment history (2 week pre-treatment with 50 mg/kg/day pyraclostrobin) had no apparent effect on metabolic disposition. The most abundant fecal metabolite was 500M08 (de-methoxylated ai, which is hydroxylated in the 4-position of the pyrazole ring), accounting for about 38% of total administered dose. Other significant fecal metabolites were further hydroxylated: usually on the chlorophenyl ring and sometimes also on the tolyl ring. The major biliary metabolite was 500M46 (formed by hydroxylation followed by glucuronidation of carbon 4 of the pyrazole group of the ai). The majority of lesser biliary metabolites were also glucuronides. No single urinary metabolite comprised more than about 3% of administered dose. Predominant urinary metabolites were various products of cleavage of the ether oxygen (often to form a glucuronide or benzoic acid derivative), or 500M06 (de-methoxylated 500M46). Detectable plasma residues were limited to 500M06 and 500M46 (representing about 0.02% of administered dose). These metabolites plus parent pyraclostrobin were found in liver in higher amounts (these 3 residues combined representing about 0.5% of dose). Only pyraclostrobin could be detected in kidneys, to the extent of about 0.03% of dose. Thus absorbed pyraclostrobin is efficiently metabolized to polar products and is cleared effectively from the body. Metabolite /is/ methyl-N-(((1- (4-chlorophenyl) pyrazol-3-yl)oxy]otolyl) carbamate (BF 500-3) Major routes of metabolism involved demethoxylation and hydroxylation of the pyrazole and other ring systems followed by glucuronidation. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Data
LC50 (rat) > 310 mg/m3/4h < 1,070 mg/m3/4h Non-Human Toxicity Values LC50 Rat (Wistar male & female) dermal >2000 mg/kg bw (no deaths) LC50 Rat (Wistar male & female) inhalation (head and nose only), 4 hr >0.310 mg/L, <1.070 mg/L LD50 Rat (Wistar male & female) oral >5000 mg/kg bw (no deaths) |
| 参考文献 |
[1]. Anthony L Luz,et al. The High-Production Volume Fungicide Pyraclostrobin Induces Triglyceride Accumulation Associated With Mitochondrial Dysfunction, and Promotes Adipocyte Differentiation Independent of PPARγ Activation, in 3T3-L1 Cells. 2018 Jan 15;393:
[2]. Alexander H Tuttle, et al.Choice of Vehicle Affects Pyraclostrobin Toxicity in Mice.Chemosphere. 2019 Mar;218:501-506. |
| 其他信息 |
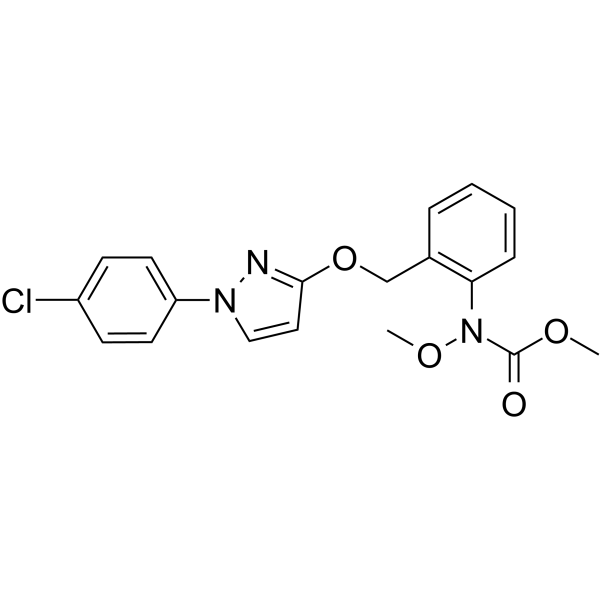
Pyraclostrobin is a carbamate ester that is the methyl ester of [2-({[1-(4-chlorophenyl)-1H-pyrazol-3-yl]oxy}methyl)phenyl]methoxycarbamic acid. A fungicide used to control major plant pathogens including Septoria tritici, Puccinia spp. and Pyrenophora teres. It has a role as a mitochondrial cytochrome-bc1 complex inhibitor, a xenobiotic, an environmental contaminant and an antifungal agrochemical. It is a member of pyrazoles, a carbamate ester, an aromatic ether, a member of monochlorobenzenes, a methoxycarbanilate strobilurin antifungal agent and a carbanilate fungicide.
Pyraclostrobin has been reported in Ganoderma lucidum with data available. Pyraclostrobin is a broad spectrum foliar fungicide belonging to the strobilurin chemical class. It acts by inhibition of mitochondrial respiration. This leads to a reduction of the available ATP quantity in the fungal cell. It is used for control or suppression of fungal diseases on many common crops including: Berries, Bulb, Cucurbit, Fruiting, and Root vegetables, and Cherries Mechanism of Action Pyraclostrobin is a member of the strobilurin group of fungicides. The strobilurin fungicides act through inhibition of mitochondrial respiration by blocking electron transfer within the respiratory chain, which in turn causes important cellular biochemical processes to be severely disrupted, and results in cessation of fungal growth. |
| 分子式 |
C19H18CLN3O4
|
|---|---|
| 分子量 |
387.82
|
| 精确质量 |
387.098
|
| CAS号 |
175013-18-0
|
| PubChem CID |
6422843
|
| 外观&性状 |
Off-white to light yellow solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
501.1±60.0 °C at 760 mmHg
|
| 熔点 |
63.7-65.2°
|
| 闪点 |
256.8±32.9 °C
|
| 蒸汽压 |
0.0±1.3 mmHg at 25°C
|
| 折射率 |
1.592
|
| LogP |
4.25
|
| tPSA |
65.82
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
476
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
HZRSNVGNWUDEFX-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H18ClN3O4/c1-25-19(24)23(26-2)17-6-4-3-5-14(17)13-27-18-11-12-22(21-18)16-9-7-15(20)8-10-16/h3-12H,13H2,1-2H3
|
| 化学名 |
methyl N-[2-[[1-(4-chlorophenyl)pyrazol-3-yl]oxymethyl]phenyl]-N-methoxycarbamate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 100 mg/mL (257.85 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.36 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.36 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5785 mL | 12.8926 mL | 25.7852 mL | |
| 5 mM | 0.5157 mL | 2.5785 mL | 5.1570 mL | |
| 10 mM | 0.2579 mL | 1.2893 mL | 2.5785 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。