| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
β-lactam
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Not absorbed following oral administration. As with other penicillins, PIPRACIL is eliminated primarily by glomerular filtration and tubular secretion; it is excreted rapidly as unchanged drug in high concentrations in the urine. Because PIPRACIL is excreted by the biliary route as well as by the renal route, it can be used safely in appropriate dosage in patients with severely restricted kidney function. 101 mL/kg [intravenous administration of 50 mg/kg (5-minute infusion) in neonates] 32 - 41 mL/min/1.73 m2 124 - 160 mL/min/1.73 m2 [older pediatric patients] Metabolism / Metabolites Largely not metabolized. Biological Half-Life 36-72 minutes |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Patients on intravenous piperacillin may have transient and mild-to-moderate serum aminotransferase elevations in up to 12% of patients, but these are of little clinical significance and not more common than with comparative parenteral antibiotics. Hepatic injury was more commonly reported with mezlocillin, a related extended spectrum ureidopenicillin which has been withdrawn from use. Rare instances of idiosyncratic liver injury have been reported in persons receiving piperacillin. The liver injury is typically cholestatic arising within 1 to 6 weeks of starting therapy. The injury can be severe, but is generally self-limited once piperacillin is stopped. The features of the hepatotoxicity resemble those of other penicillins. The cholestatic hepatitis caused by piperacillin and other penicillins can be prolonged and lead to persistent cholestasis (vanishing bile duct syndrome) or persistent elevations in serum alkaline phosphatase suggestive of partial bile duct loss. Most cases of liver injury related to piperacillin are linked to the combination of piperacillin with the beta-lactamase inhibitor tazobactam (Zosyn and generics), which is more commonly used than piperacillin alone. Likelihood score: B (known rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited information indicates that piperacillin produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with penicillins, but these effects have not been adequately evaluated. Piperacillin is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| 参考文献 |
[1]. B. Holmes, et al. A Review of its Antibacterial Activity, Pharmacokinetic Properties and Therapeutic Use.
|
| 其他信息 |
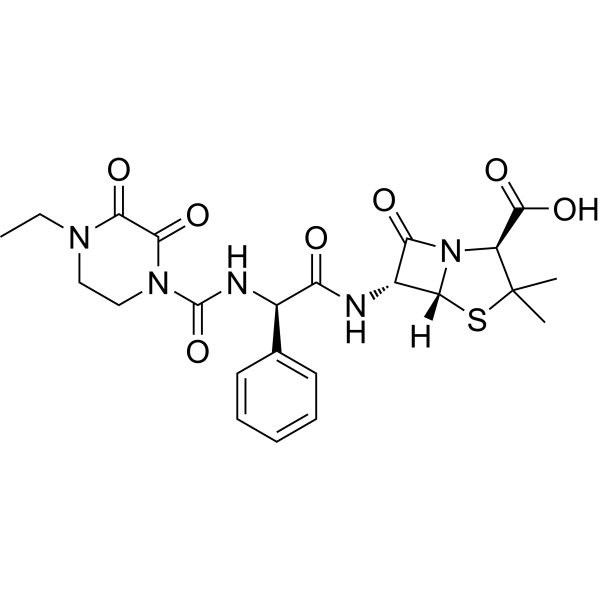
Piperacillin is a penicillin in which the substituent at position 6 of the penam ring is a 2-[(4-ethyl-2,3-dioxopiperazin-1-yl)carboxamido]-2-phenylacetamido group. It has a role as an antibacterial drug. It is a penicillin and a penicillin allergen. It is a conjugate acid of a piperacillin(1-).
Semisynthetic, broad-spectrum, ampicillin derived ureidopenicillin antibiotic proposed for pseudomonas infections. It is also used in combination with other antibiotics. Piperacillin anhydrous is a Penicillin-class Antibacterial. Piperacillin is an extended spectrum ureidopenicillin and is used to treat moderate-to-severe infections due to susceptible organisms. Piperacillin has been linked with idiosyncratic liver injury, but only rarely and in isolated case reports. Piperacillin has been reported in Apis cerana with data available. Piperacillin Anhydrous is the anhydrous form of piperacillin, a broad-spectrum semisynthetic ureidopenicillin antibiotic. Piperacillin binds to penicillin binding proteins (PBP) located on the inner membrane of the bacterial cell wall, thereby interfering with the cross-linking of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. As a result, cell wall synthesis is interrupted leading to a weakened cell wall and eventually cell lysis. Piperacillin is a broad-spectrum semisynthetic, ampicillin-derived ureidopenicillin antibiotic. Piperacillin binds to penicillin binding proteins (PBP), the enzymes that catalyze the synthesis of peptidoglycan, a critical component of the bacterial cell wall. This blockade leads to the interruption of cell wall synthesis, consequently, leading to bacterial cell growth inhibition and cell lysis. Semisynthetic, broad-spectrum, AMPICILLIN derived ureidopenicillin antibiotic proposed for PSEUDOMONAS infections. It is also used in combination with other antibiotics. See also: Piperacillin Sodium (annotation moved to). Drug Indication For the treatment of polymicrobial infections. Mechanism of Action By binding to specific penicillin-binding proteins (PBPs) located inside the bacterial cell wall, Piperacillin inhibits the third and last stage of bacterial cell wall synthesis. Cell lysis is then mediated by bacterial cell wall autolytic enzymes such as autolysins; it is possible that Piperacillin interferes with an autolysin inhibitor. |
| 分子式 |
C23H27N5O7S
|
|---|---|
| 分子量 |
517.55
|
| 精确质量 |
517.163
|
| CAS号 |
61477-96-1
|
| 相关CAS号 |
Piperacillin sodium;59703-84-3;Piperacillin-d5
|
| PubChem CID |
43672
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 熔点 |
139-140ºC
|
| 折射率 |
1.678
|
| LogP |
1.88
|
| tPSA |
181.73
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
36
|
| 分子复杂度/Complexity |
982
|
| 定义原子立体中心数目 |
4
|
| SMILES |
CCN1CCN(C(=O)C1=O)C(=O)N[C@H](C2=CC=CC=C2)C(=O)N[C@H]3[C@@H]4N(C3=O)[C@H](C(S4)(C)C)C(=O)O
|
| InChi Key |
IVBHGBMCVLDMKU-GXNBUGAJSA-N
|
| InChi Code |
InChI=1S/C23H27N5O7S/c1-4-26-10-11-27(19(32)18(26)31)22(35)25-13(12-8-6-5-7-9-12)16(29)24-14-17(30)28-15(21(33)34)23(2,3)36-20(14)28/h5-9,13-15,20H,4,10-11H2,1-3H3,(H,24,29)(H,25,35)(H,33,34)/t13-,14-,15+,20-/m1/s1
|
| 化学名 |
(2S,5R,6R)-6-[[(2R)-2-[(4-ethyl-2,3-dioxopiperazine-1-carbonyl)amino]-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 100 mg/mL (193.22 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.83 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.83 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.83 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9322 mL | 9.6609 mL | 19.3218 mL | |
| 5 mM | 0.3864 mL | 1.9322 mL | 3.8644 mL | |
| 10 mM | 0.1932 mL | 0.9661 mL | 1.9322 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。