| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Broad-spectrum antiviral
|
|---|---|
| 体外研究 (In Vitro) |
严重急性呼吸综合征冠状病毒2的RNA依赖性RNA聚合酶是当前治疗2019冠状病毒病药物开发工作的重要靶点。莫努匹拉韦是一种广谱抗病毒药物,是核苷类似物β-D-N4-羟基胞苷(NHC)的口服生物可利用前药。Molnupiravir或NHC可以增加复制冠状病毒的G到A和C到U过渡突变。突变频率的增加可能与抗病毒作用的增加有关;然而,莫努匹拉韦诱导突变的生化数据尚未报道。在这里,我们研究了活性化合物NHC5'-三磷酸(NHC-TP)对纯化的严重急性呼吸综合征冠状病毒2 RNA依赖性RNA聚合酶复合物的作用。天然核苷酸的掺入效率高于NHC-TP掺入模型RNA底物的效率,其顺序为GTP(12841)>ATP(424)>UTP(171)>CTP(30),表明NHC-TP主要与CTP竞争掺入。由于在RNA引物链中掺入一磷酸,没有观察到对RNA合成的显著抑制。当嵌入模板链中时,一磷酸NHC以相似的效率支持NHC:G和NHC:A碱基对的形成。NHC:G产物的延伸受到适度抑制,但较高的核苷酸浓度可以克服这种阻碍。相反,NHC:A碱基对导致观察到的G到A(G:NHC:A)或C到U(C:G:NHC:A:U)突变。总之,这些生物化学数据支持莫努匹拉韦的作用机制,该机制主要基于通过模板链介导的RNA诱变[3]。
|
| 体内研究 (In Vivo) |
Molnupiravir 具有很强的抗病毒特性,可以阻止 SARS-CoV 的增殖和疾病 [1]。每 12 小时口服一次,持续三天,剂量为 50-500 mg/kg。每天口服两次莫努匹拉韦 (molnupiravir) (7 mg/kg),持续 3.5 天,可显着减少发烧持续时间和病毒载量 [2]。
|
| 酶活实验 |
NTP掺入和包埋引物或模板的NHC-MP对病毒RNA合成的影响[3]
根据我们的报告,通过严重急性呼吸系统综合征冠状病毒2型RdRp掺入NTP,并进行数据采集和定量。单核苷酸和多核苷酸掺入测定的酶浓度分别为100或200 nM。RNA合成孵育时间为10分钟。使用来自单核苷酸掺入测定的数据来确定天然核苷酸相对于NHC-TP的偏好。选择性值计算为天然核苷酸与核苷酸类似物的结合效率之比。核苷酸掺入的效率由Michaelis–Menten常数Vmax与Km的比值决定。核苷酸掺入底物是通过将[α-32P]NTP掺入4-nt引物而产生的5-nt引物。5-nt引物的形成在给定的时间点是最大的;然而,它的确切浓度是未知的。因此,通过量化对应于6-nt引物产物的信号并将其除以反应中的总信号(5-nt引物和6-nt引物)来测量反应中产生的产物。这定义了产品分数。产物分数通常乘以总底物浓度,以确定Vmax的摩尔单位,这在这里是不可能的,如上所述。因此,Vmax的单位被报告为随时间变化的乘积分数。选择性值是无单位的,因为它是具有相同单位的两个Vmax/Km测量的比率。如我们所述,制备了具有嵌入NHC-MP的RNA模板。图S1中解释了与NHC相关的方案修改。
|
| 细胞实验 |
Madin-Darby犬肾(MDCK)细胞(ATCC CCL-34)在补充有7.5%胎牛血清(FBS)的Dulbecco改良Eagle培养基(DMEM)中于37°C和5%CO2下生长。来自30岁健康女性供体的正常原代人支气管气管上皮细胞(HBTECs)在支气管生命细胞培养基中生长。这些细胞由供应商在知情同意的情况下获得,并符合赫尔辛基宣言、《人体组织法》(英国)、CFR第21篇和HIPAA法规。所有监管审批均由供应商负责。本研究中使用的永生细胞系定期检查微生物污染(间隔约6个月)。2017年7月25日,LifeLine Cell Technology对HBTEC进行了微生物污染测试。本研究仅使用了通道编号为1-4的重型作战电子计算机[2]。
|
| 动物实验 |
Animal/Disease Models: C57BL/6 mice (intranasal infection with SARS-CoV)[1]
Doses: 50, 150, 500 mg/kg Route of Administration: Oral; every 12 hrs (hours) for 3 days Experimental Results: Body weight loss is Dramatically diminished or prevented. Animal/Disease Models: Ca/09-infected female ferrets[1] Doses: 7 mg/kg Route of Administration: Oral; twice (two times) daily for 3.5 days Experimental Results: Shed virus load and duration of fever were Dramatically diminished. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
After an 800 mg oral dose of molnupiravir every 12 hours, the active compound (N4-hydroxycytidine) reaches a Cmax of 2970 ng/mL, with a Tmax of 1.5 hours, and an AUC0-12h of 8360 h\*ng/mL. ≤3% of an oral molnupiravir dose is eliminated in the urine as the active metabolite N4-hydroxycytidine. Metabolism / Metabolites Molnupiravir is hydrolyzed to [N4-hydroxycytidine], which distributes into tissues. Once inside cells, N4-hydroxycytidine is phosphorylated to the 5'-triphosphate form. Biological Half-Life The half life of the active metabolite, N4-hydroxycytidine, is 3.3 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In preregistration clinical trials, serum aminotransferase elevations were uncommon and mild, and were no more frequent with molnupiravir than with placebo. Furthermore, among more than 900 patients treated with molnupiravir (800 mg twice daily) for 5 days in prelicensure studies, there were no reported episodes of clinically apparent liver injury. Confounding the issue is that serum aminotransferase elevations are common during symptomatic SARS-CoV-2 infection, present in up to 70% of patients and are more frequent in patients with severe disease and in those with the known risk factors for COVID-19 severity such as male sex, older age, higher body mass index and diabetes. Thus, molnupiravir has not been shown to cause liver injury, but the total clinical experience with its use is limited. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of molnupiravir during breastfeeding. Breastfeeding is not recommended during treatment and for 4 days after the last dose of molnupiravir. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. ◈ What is molnupiravir? Molnupiravir is an antiviral medication that has been given emergency permission by the U.S. Food and Drug Administration (FDA) to treat mild to moderate COVID-19 in certain patients. Molnupiravir must be started within 5 days of having symptoms of COVID-19 in order to be effective. A brand name for molnupiravir is Lagevrio®.The FDA emergency use guidelines for molnupiravir recommend people who are pregnant not use this medication unless there are no other treatment options and treatment is clearly needed. This is because there is not enough information available on the use of molnupiravir to know if/how it could affect a pregnancy. However, the benefit of using molnupiravir may outweigh possible risks. Your healthcare provider can talk with you about using molnupiravir and what treatment is best for you. For more information about COVID-19, please the see the MotherToBaby fact sheet at https://mothertobaby.org/fact-sheets/covid-19/. ◈ I am taking molnupiravir, but I would like to get pregnant after taking it. How long does the drug stay in my body? People eliminate medication at different rates. In non-pregnant adults, it takes up to 1 day, on average, for most of the molnupiravir to be gone from the body. The FDA emergency use guidelines recommend that females avoid trying to get pregnant while they are taking molnupiravir and for 4 days after the last dose. ◈ I take molnupiravir. Can it make it harder for me to get pregnant? It is not known if molnupiravir can make it harder to get pregnant. The FDA emergency use guidelines recommend that females who can get pregnant use effective contraception correctly and consistently while they are taking molnupiravir and for 4 days after the last dose. ◈ Does taking molnupiravir increase the chance of miscarriage? Miscarriage is common and can occur in any pregnancy for many different reasons. Studies have not been done in humans to see if molnupiravir can increase the chance of miscarriage. ◈ Does taking molnupiravir increase the chance of birth defects? Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Studies have not been done in humans to see if molnupiravir can increase the chance of birth defects above the background risk. ◈ Does taking molnupiravir in pregnancy increase the chance of other pregnancy-related problems? Studies have not been done in humans to see if molnupiravir can increase the chance of pregnancy-related problems such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth). Having COVID-19 during pregnancy can increase the chance of preterm delivery, stillbirth, and other pregnancy complications. ◈ Does taking molnupiravir in pregnancy affect future behavior or learning for the child? Studies have not been done to see if molnupiravir can cause behavior or learning issues for the child. ◈ Breastfeeding while taking molnupiravir: The FDA emergency use guidelines for molnupiravir recommend that people who are breastfeeding not use this medication unless there are no other treatment options and treatment is clearly needed. But the benefit of using molnupiravir along with the benefits of breastfeeding your baby may outweigh possible risks. People who are breastfeeding can consider pumping and discarding breast milk during treatment with molnupiravir and for 4 days after the last dose. Your healthcare providers can talk with you about using molnupiravir and what treatment is best for you. Be sure to talk to your healthcare provider about all your breastfeeding questions. ◈ If a male takes molnupiravir, could it affect fertility or increase the chance of birth defects? Studies have not been done to see if molnupiravir could affect male fertility (ability to get partner pregnant) or increase the chance of birth defects above the background risk. The FDA emergency use guidelines recommend that males use a reliable method of contraception correctly and consistently during treatment and for at least 3 months after the last dose of molnupiravir. In general, exposures that fathers or sperm donors have are unlikely to increase risks to a pregnancy. For more information, please see the MotherToBaby fact sheet Paternal Exposures at https://mothertobaby.org/fact-sheets/paternal-exposures-pregnancy/. Protein Binding Molnupiravir and the active metabolite, N4-hydroxycytidine, are not protein bound in plasma. |
| 参考文献 |
[1]. Sheahan TP, et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020 Apr 6. pii: eabb5883.
[2]. Toots M, et al. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci Transl Med. 2019 Oct 23;11(515). pii: eaax5866. [3]. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. Biol Chem. 2021 Jul; 297(1): 100770. |
| 其他信息 |
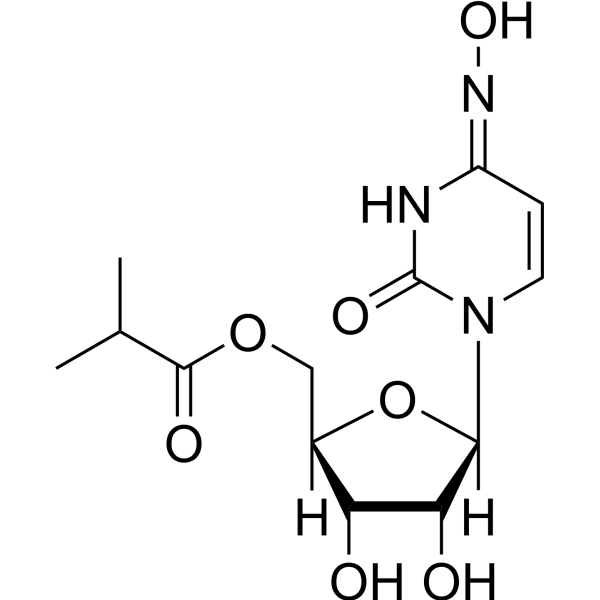
Molnupiravir is a nucleoside analogue that is N(4)-hydroxycytidine in which the 5'-hydroxy group is replaced by a (2-methylpropanoyl)oxy group. It is the prodrug of the active antiviral ribonucleoside analog N(4)-hydroxycytidine (EIDD-1931), has activity against a number of RNA viruses including SARS-CoV-2, MERS-CoV, and seasonal and pandemic influenza viruses. It is currently in phase III trials for the treatment of patients with COVID-19. It has a role as a prodrug, an anticoronaviral agent and an antiviral drug. It is a nucleoside analogue, an isopropyl ester and a ketoxime. It is functionally related to a N(4)-hydroxycytidine.
Molnupiravir (EIDD-2801, MK-4482) is the isopropylester prodrug of [N4-hydroxycytidine]. With improved oral bioavailability in non-human primates, it is hydrolyzed in vivo, and distributes into tissues where it becomes the active 5’-triphosphate form. The active drug incorporates into the genome of RNA viruses, leading to an accumulation of mutations known as viral error catastrophe. Recent studies have shown molnupiravir inhibits replication of human and bat coronaviruses, including SARS-CoV-2, in mice and human airway epithelial cells. A [remdesivir] resistant mutant mouse hepatitis virus has also been shown to have increased sensitivity to N4-hydroxycytidine. Molnupiravir was granted approval by the UK's Medicines and Health products Regulatory Agency (MHRA) on 4 November 2021 to prevent severe outcomes such as hospitalization and death due to COVID-19 in adults. Molnupiravir was also granted emergency use authorization by the FDA on December 23, 2021; however, it is not yet fully approved. Molnupiravir is a ribonucleoside analogue and antiviral agent that is used in the therapy the severe acute respiratory syndrome (SARS) coronavirus 2 (CoV-2) infection, the cause of the novel coronavirus disease, 2019 (COVID-19). Molnupiravir therapy is given orally for 5 days early in the course of SARS-CoV-2 infection and has not been linked to serum aminotransferase elevations or to clinically apparent liver injury. Molnupiravir is an orally bioavailable prodrug of EIDD-1931, the synthetic ribonucleoside derivative N4-hydroxycytidine and ribonucleoside analog, with potential antiviral activity against a variety of RNA viruses. Upon oral administration, molnupiravir, being a prodrug, is metabolized into its active form EIDD-1931 and converted into its triphosphate (TP) form. The TP form of EIDD-1931 is incorporated into RNA and inhibits the action of viral RNA-dependent RNA polymerase. This results in the termination of RNA transcription and decreases viral RNA production, and viral RNA replication. Drug Indication [N4-hydroxycytidine] and its prodrug molnupiravir are being studied for its activity against a number of viral infections including influenza, MERS-CoV, and SARS-CoV-2. Molnupiravir is approved in the UK for reducing the risk of hospitalization and death in mild to moderate COVID-19 cases for patients at increased risk of severe disease (eg. with obesity, diabetes mellitus, heart disease, or are over 60 years old). In the US, molnupiravir is authorized for emergency use for the treatment of high-risk adults With mild to moderate COVID-19. Prevention of Coronavirus disease 2019 (COVID-19) Treatment of Coronavirus disease 2019 (COVID-19) Mechanism of Action Molnupiravir is hydrolyzed _in vivo_ to N4-hydroxycytidine, which is phosphorylated in tissue to the active 5’-triphosphate form, and incorporated into the genome of new virions, resulting in the accumulation of inactivating mutations, known as viral error catastrophe. A [remdesivir] resistant mutant mouse hepatitis virus has also been shown to have increased sensitivity to N4-hydroxycytidine. |
| 分子式 |
C13H19N3O7
|
|---|---|
| 分子量 |
329.3059
|
| 精确质量 |
329.12
|
| 元素分析 |
C, 47.42; H, 5.82; N, 12.76; O, 34.01
|
| CAS号 |
2492423-29-5
|
| 相关CAS号 |
Molnupiravir-d7
|
| PubChem CID |
145996610
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
-0.8
|
| tPSA |
141Ų
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
23
|
| 分子复杂度/Complexity |
534
|
| 定义原子立体中心数目 |
4
|
| SMILES |
O1[C@]([H])([C@@]([H])([C@@]([H])([C@@]1([H])C([H])([H])OC(C([H])(C([H])([H])[H])C([H])([H])[H])=O)O[H])O[H])N1C(N=C(C([H])=C1[H])N([H])O[H])=O
|
| InChi Key |
O[C@@H]([C@H]([C@H](N1C(N/C(C=C1)=N/O)=O)O2)O)[C@H]2COC(C(C)C)=O
|
| InChi Code |
HTNPEHXGEKVIHG-QCNRFFRDSA-N
|
| 化学名 |
InChI=1S/C13H19N3O7/c1-6(2)12(19)22-5-7-9(17)10(18)11(23-7)16-4-3-8(15-21)14-13(16)20/h3-4,6-7,9-11,17-18,21H,5H2,1-2H3,(H,14,15,20)/t7-,9-,10-,11-/m1/s1
|
| 别名 |
MK 4482; EIDD-2801; EIDD 2801; Molnupiravir; MK-4482; MK4482;
EIDD2801; prodrug-EIDD-1931; prodrug-EIDD 1931; prodrug-EIDD1931.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 50 mg/mL (151.83 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 12.05 mg/mL (36.59 mM) in 10% PEG400 2.5% Ethoxylated hydrogenated castor oil 87.5% water (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
配方 2 中的溶解度: ≥ 2.5 mg/mL (7.59 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (7.59 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.5 mg/mL (7.59 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,您可以将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0367 mL | 15.1833 mL | 30.3665 mL | |
| 5 mM | 0.6073 mL | 3.0367 mL | 6.0733 mL | |
| 10 mM | 0.3037 mL | 1.5183 mL | 3.0367 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。