| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
Ferroptosis (IC50 = 22 nM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
在小鼠胚胎成纤维细胞中,lipostatin-1 具有抗铁死亡作用,IC50 约为 38 nM[2]。Fer1和Lip-1 (lipostatin-1)天生是好的,但不是很好的,自由基捕获抗氧化剂;fe -1和Lip-1是磷脂双层中优异的自由基捕获抗氧化剂fer1和Lip-1充其量是15-LOX-1的不良抑制剂,α-TOH也是如此。[2] <人力资源>
Lipostatin-1 治疗能够保护HRPTEpiCs免受rsl3诱导的细胞死亡,这支持了铁凋亡的参与。在永生化的人肾近端小管上皮细胞系HK-2中也获得了类似的结果。接下来,我们使用sirna库敲除HK-2细胞中的Gpx4,揭示了对αToc处理敏感的细胞活力虽小但显著下降(补充图7c)。然而,由于Gpx4在肾小管上皮细胞中的高表达水平,通过敲低Gpx4诱导细胞死亡被证明是具有挑战性的(补充图7d)。尽管如此,Gpx4敲低使细胞对铁致凋亡诱导剂更敏感(补充图7e),表明Gpx4调节的铁致凋亡机制在人类近端小管上皮细胞中起作用。此外,rsl3诱导的BODIPY 581/591 C11氧化可以被Liproxstatin-1阻断(图7b),这表明Liproxstatin-1也可以阻止人类铁致细胞死亡[1]。
|
||
| 体内研究 (In Vivo) |
在人类细胞、Gpx4/肾脏和缺血/再灌注诱导的组织损伤模型中,liprostatin-1(10 mg/kg,腹腔注射)可减少铁死亡[1]。
接下来,研究人员评估了liprostatin-1在动物体内预防诱导性Gpx4破坏后果的潜力。在对CreERT2;Gpx4fl/fl小鼠进行TAM治疗时,小鼠每天腹腔注射liprostatin-1,直到小鼠出现急性肾功能衰竭(ARF)的迹象,此时它们被安乐死(图7c)。值得注意的是,与药物治疗组相比,liprostatin-1显著延长了生存期。TAM处理后第9天的TUNEL染色显示,与药物处理组相比,liprostatin-1中TUNEL+细胞数量明显减少(图7d),表明liprostatin-1延缓了小管细胞的铁凋亡。图1c中ARF引起的小鼠死亡与图7c中药物处理动物死亡的差异可以用TAM给药方式来解释,即喂食与注射。 作为一项独立的概念验证,我们分析了liprostatin-1在肝脏缺血/再灌注损伤真实模型中的体内疗效,提供了liprostatin-1减轻缺血/再灌注肝损伤组织损伤的证据(图7e)。因此,这些数据暗示铁下垂是缺血/再灌注诱导的组织损伤的一个因素,并为治疗相关病理的治疗方法的开发带来了巨大的希望。[1] |
||
| 酶活实验 |
苯乙烯的自氧化抑制[2]
这些实验是以类似于我们在以前的工作中所描述的方式进行的简而言之,苯乙烯用1m NaOH水溶液洗涤三次,在MgSO4上干燥,过滤,真空蒸馏,通过二氧化硅渗透纯化,然后是碱性氧化铝。在1.25 mL苯乙烯的比色皿中加入1.18 mL氯苯,在37℃下平衡5min。空白试管,在1,2,4-三氯苯中加入12.5 μL 2 mM PBD-BODIPY,再加入50 μL 0.3 M氯苯AIBN,充分混合。20min后,加入liprostatin-1、fe -1、C15-THN、PMHC或α-TOH原液(1mm)的氯苯溶液,在591 nm处失去吸光度。每个实验的抑制速率常数(kinh)和化学计量(n)根据图1B确定(完整细节见辅助信息)。在每个浓度下进行三次技术重复的自氧化,动力学以平均值±标准差报告。 抑制PC脂质体的自氧化作用[2] 在2.34 mL pH为7.4的10 mM PBS培养皿中加入脂质体(pH为7.4的20 mM PBS中125 μL),在37℃下平衡5min。空白试管,在DMSO中加入10 μL 2 mM的STY-BODIPY,然后在乙腈中加入10 μL 0.05 M的MeOAMVN,充分混合。5 min后,加入liprostatin-1、fe -1、C15-THN、PMHC或α-TOH原液(1 mM),在DMSO中测定565 nm处吸光度损失。根据图3B测定每个实验的抑制速率常数(kinh)和化学计量学(n)(见附图(完整细节见附图))。在每个浓度下进行三次技术重复的自氧化,动力学以平均值±标准差报告。在脂质体挤出前添加抗氧化剂的选择对照实验中获得了难以区分的结果。 |
||
| 细胞实验 |
Ferroptosis抑制剂的表型筛选[1]
简而言之,将化合物播种到96孔板上(每孔1000个细胞),同时给药1 μM TAM(导致Gpx4失活),避免多次更换培养基,然后孵育72小时。随后使用活/死实验染料AquaBluer评估细胞活力。在第一轮筛选中,所有化合物在10 μM的单一浓度下进行测试,并从细胞存活率>80%的井中选择阳性命中。为了确认最初的命中,在相同的实验中重新筛选化合物,并在0-100 μM的浓度下获得剂量依赖性生存和毒性曲线。采用GraphPad Prism软件计算IC50和TC50值。然后根据疗效、对ferroptosis的选择性、治疗范围和物理化学性质对验证命中进行评估。此外,还进行了计算机ADME-Tox筛选,以排除具有潜在体内副作用的化合物。为了进一步验证liprostatin-1,使用市售衍生物进行了SAR研究。 |
||
| 动物实验 |
|
||
| 参考文献 |
|
||
| 其他信息 |
Ferroptosis is a non-apoptotic form of cell death induced by small molecules in specific tumour types, and in engineered cells overexpressing oncogenic RAS. Yet, its relevance in non-transformed cells and tissues is unexplored and remains enigmatic. Here, we provide direct genetic evidence that the knockout of glutathione peroxidase 4 (Gpx4) causes cell death in a pathologically relevant form of ferroptosis. Using inducible Gpx4(-/-) mice, we elucidate an essential role for the glutathione/Gpx4 axis in preventing lipid-oxidation-induced acute renal failure and associated death. We furthermore systematically evaluated a library of small molecules for possible ferroptosis inhibitors, leading to the discovery of a potent spiroquinoxalinamine derivative called Liproxstatin-1, which is able to suppress ferroptosis in cells, in Gpx4(-/-) mice, and in a pre-clinical model of ischaemia/reperfusion-induced hepatic damage. In sum, we demonstrate that ferroptosis is a pervasive and dynamic form of cell death, which, when impeded, promises substantial cytoprotection.[1]
Ferroptosis is a form of regulated necrosis associated with the iron-dependent accumulation of lipid hydroperoxides that may play a key role in the pathogenesis of degenerative diseases in which lipid peroxidation has been implicated. High-throughput screening efforts have identified ferrostatin-1 (Fer-1) and liproxstatin-1 (Lip-1) as potent inhibitors of ferroptosis - an activity that has been ascribed to their ability to slow the accumulation of lipid hydroperoxides. Herein we demonstrate that this activity likely derives from their reactivity as radical-trapping antioxidants (RTAs) rather than their potency as inhibitors of lipoxygenases. Although inhibited autoxidations of styrene revealed that Fer-1 and Lip-1 react roughly 10-fold more slowly with peroxyl radicals than reactions of α-tocopherol (α-TOH), they were significantly more reactive than α-TOH in phosphatidylcholine lipid bilayers - consistent with the greater potency of Fer-1 and Lip-1 relative to α-TOH as inhibitors of ferroptosis. None of Fer-1, Lip-1, and α-TOH inhibited human 15-lipoxygenase-1 (15-LOX-1) overexpressed in HEK-293 cells when assayed at concentrations where they inhibited ferroptosis. These results stand in stark contrast to those obtained with a known 15-LOX-1 inhibitor (PD146176), which was able to inhibit the enzyme at concentrations where it was effective in inhibiting ferroptosis. Given the likelihood that Fer-1 and Lip-1 subvert ferroptosis by inhibiting lipid peroxidation as RTAs, we evaluated the antiferroptotic potential of 1,8-tetrahydronaphthyridinols (hereafter THNs): rationally designed radical-trapping antioxidants of unparalleled reactivity. We show for the first time that the inherent reactivity of the THNs translates to cell culture, where lipophilic THNs were similarly effective to Fer-1 and Lip-1 at subverting ferroptosis induced by either pharmacological or genetic inhibition of the hydroperoxide-detoxifying enzyme Gpx4 in mouse fibroblasts, and glutamate-induced death of mouse hippocampal cells. These results demonstrate that potent RTAs subvert ferroptosis and suggest that lipid peroxidation (autoxidation) may play a central role in the process.[2] |
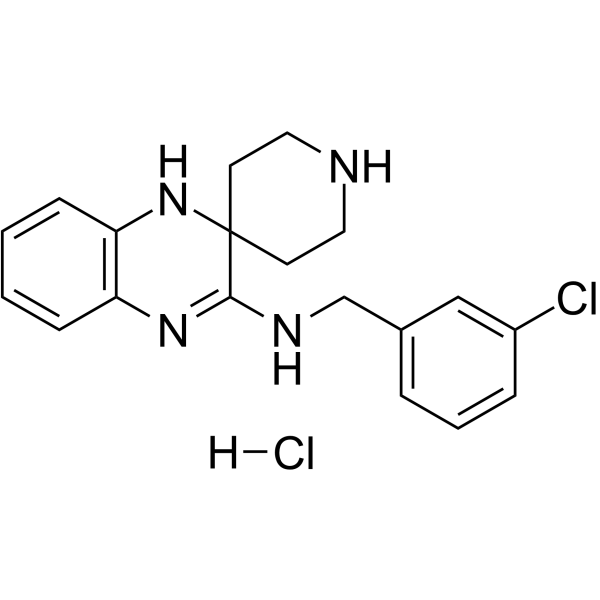
| 分子式 |
C19H22CL2N4
|
|---|---|
| 分子量 |
377.310781955719
|
| 精确质量 |
376.122
|
| CAS号 |
2250025-95-5
|
| 相关CAS号 |
Liproxstatin-1;950455-15-9;Liproxstatin-1-13C6;Liproxstatin-1-15N
|
| PubChem CID |
136590563
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| tPSA |
48.4
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
25
|
| 分子复杂度/Complexity |
460
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
HEHOHTKMIOBTKC-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H21ClN4.ClH/c20-15-5-3-4-14(12-15)13-22-18-19(8-10-21-11-9-19)24-17-7-2-1-6-16(17)23-18;/h1-7,12,21,24H,8-11,13H2,(H,22,23);1H
|
| 化学名 |
N-[(3-chlorophenyl)methyl]spiro[1,4-dihydroquinoxaline-3,4'-piperidine]-2-imine;hydrochloride
|
| 别名 |
Liproxstatin-1 (hydrochloride); 2250025-95-5; Liproxstatin-1 hydrochloride; HY-12726A; AKOS034834095; CS-0120787; Liproxstatin-1 HCl (950455-15-9 free base); N-(3-Chlorobenzyl)-1'H-spiro[piperidine-4,2'-quinoxalin]-3'-amine hydrochloride;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6503 mL | 13.2517 mL | 26.5034 mL | |
| 5 mM | 0.5301 mL | 2.6503 mL | 5.3007 mL | |
| 10 mM | 0.2650 mL | 1.3252 mL | 2.6503 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。