| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
In healthy subjects, the pharmacokinetics of isavuconazole following oral administration of isavuconazonium capsules at isavuconazole equivalent doses up to 600 mg per day (6 capsules) are dose-proportional. Following oral administration of isavuconazonium capsules at an isavuconazole equivalent dose of 200 mg in 66 fasted healthy male subjects, a single dose administration of two 186 mg isavuconazonium capsules and five 74.5 mg isavuconazonium capsules exhibited a mean (SD) Cmax and AUC of 3.3 (0.6) mg/L and 112.2 (30.3) mg·hr/L, respectively, and 3.3 (0.6) mg/L and 118.0 (33.1) mg·hr/L, respectively. After oral administration of isavuconazonium in healthy volunteers, the active moiety, isavuconazole, generally reaches maximum plasma concentrations (Cmax) 2 hours to 3 hours after single and multiple dosing. The absolute bioavailability of isavuconazole following oral administration of isavuconazonium is 98%. No significant concentrations of the prodrug or inactive cleavage product were seen in plasma after oral administration. Following intravenous administration of isavuconazonium, maximal plasma concentrations of the prodrug and inactive cleavage product were detectable during infusion and declined rapidly following the end of administration. The prodrug was below the level of detection by 1.25 hours after the start of a one-hour infusion. The total exposure of the prodrug based on AUC was less than 1% that of isavuconazole. The inactive cleavage product was quantifiable in some subjects up to 8 hours after the start of infusion. The total exposure of inactive cleavage product based on AUC was approximately 1.3% that of isavuconazole. Isavuconazonium given orally as an intravenous solution administered via nasogastric (NG) tube provides systemic isavuconazole exposure that is similar to the oral capsule. Coadministration of isavuconazonium equivalent to isavuconazole 400 mg oral dose with a high-fat meal reduced isavuconazole Cmax by 9% and increased AUC by 9%. isavuconazonium can be taken with or without food. Following oral administration of radio-labeled isavuconazonium sulfate to healthy volunteers, a mean of 46.1% of the total radioactive dose was recovered in the feces and 45.5% was recovered in the urine. Renal excretion of isavuconazole itself was less than 1% of the dose administered. The inactive cleavage product is primarily eliminated by metabolism and subsequent renal excretion of the metabolites. Renal elimination of intact cleavage product was less than 1% of the total dose administered. Following intravenous administration of radio-labeled cleavage product, 95% of the total radioactive dose was excreted in the urine. Isavuconazole is extensively distributed with a mean steady-state volume of distribution (Vss) of approximately 450 L. In healthy subjects, the clearance of isavuconazole was estimated to be from 2.4 to 4.1 L/h. Chinese subjects were found to have on average a 40% lower clearance compared to Western subjects (1.6 L/hr for Chinese subjects as compared to 2.6 L/hr for Western subjects). Metabolism / Metabolites In in vitro studies, isavuconazonium sulfate is rapidly hydrolyzed in blood to isavuconazole by esterases, predominantly by butylcholinesterase. Isavuconazole is a substrate of cytochrome P450 enzymes 3A4 and 3A5. Following single doses of [cyano 14C] isavuconazonium and [pyridinylmethyl 14C] isavuconazonium in humans, in addition to the active moiety (isavuconazole) and the inactive cleavage product, several minor metabolites were identified. Except for the active moiety isavuconazole, no individual metabolite was observed with an AUC greater than 10% of drug-related material. In vivo studies indicate that CYP3A4, CYP3A5, and subsequently uridine diphosphate-glucuronosyltransferases (UGT) are involved in the metabolism of isavuconazole. Biological Half-Life Based on a population pharmacokinetics analysis of healthy subjects and patients, the mean plasma half-life of isavuconazole was 130 hours. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Transient elevations in serum aminotransferase levels occur in 1% to 5% of patients on isavuconazonium. These elevations are usually asymptomatic and self-limited, but occasional patients require discontinuation of isavuconazonium because of ALT elevations. Clinically apparent hepatotoxicity has not been reported with isavuconazonium, but it has had limited general use. Other triazoles, such as fluconazole and voriconazole that have been available for more than a decade and have had wide scale use, have been associated with rare instances of clinically apparent liver injury. The injury arises within the first few months of therapy and the pattern of serum enzyme elevations has been variable from cholestatic to hepatocellular. Several cases of acute liver failure attributed to other triazoles have been reported. Immunoallergic features and autoantibodies are uncommon. Recovery upon stopping therapy generally takes 6 to 10 weeks but, in some cases, the time to complete resolution may be prolonged. Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury). Protein Binding Isavuconazole is highly protein bound (greater than 99%), predominantly to albumin. |
| 参考文献 |
1: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. Isavuconazonium. 2018 Apr 27. PMID: 31643955. 2: McCormack PL. Isavuconazonium: first global approval. Drugs. 2015 May;75(7):817-22. doi: 10.1007/s40265-015-0398-6. PMID: 25902926. 3: Walker RC, Zeuli JD, Temesgen Z. Isavuconazonium sulfate for the treatment of fungal infection. Drugs Today (Barc). 2016 Jan;52(1):7-16. doi: 10.1358/dot.2016.52.1.2404002. PMID: 26937491. 4: Isavuconazonium sulfate (Cresemba)--a new antifungal. Med Lett Drugs Ther. 2016 Mar 14;58(1490):37-8. PMID: 26963156. 5: McCreary EK, Nguyen MH, Davis MR, Borlagdan J, Shields RK, Anderson AD, Rivosecchi RM, Marini RV, Sacha LM, Silveira FP, Andes DR, Lepak AJ. Achievement of clinical isavuconazole blood concentrations in transplant recipients with isavuconazonium sulphate capsules administered via enteral feeding tube. J Antimicrob Chemother. 2020 Oct 1;75(10):3023-3028. doi: 10.1093/jac/dkaa274. PMID: 32710097; PMCID: PMC7778376. 6: Murrell D, Bossaer JB, Carico R, Harirforoosh S, Cluck D. Isavuconazonium sulfate: a triazole prodrug for invasive fungal infections. Int J Pharm Pract. 2017 Feb;25(1):18-30. doi: 10.1111/ijpp.12302. Epub 2016 Aug 29. PMID: 27569742. 7: Adamsick ML, Elshaboury RH, Gift T, Mansour MK, Kotton CN, Gandhi RG. Therapeutic drug concentrations of isavuconazole following the administration of isavuconazonium sulfate capsules via gastro-jejunum tube: A case report. Transpl Infect Dis. 2019 Apr;21(2):e13048. doi: 10.1111/tid.13048. Epub 2019 Jan 29. PMID: 30636363. 8: Peyton LR, Gallagher S, Hashemzadeh M. Triazole antifungals: a review. Drugs Today (Barc). 2015 Dec;51(12):705-18. doi: 10.1358/dot.2015.51.12.2421058. PMID: 26798851. 9: Kovanda LL, Maher R, Hope WW. Isavuconazonium sulfate: a new agent for the treatment of invasive aspergillosis and invasive mucormycosis. Expert Rev Clin Pharmacol. 2016 Jul;9(7):887-97. doi: 10.1080/17512433.2016.1185361. Epub 2016 May 21. PMID: 27160418. 10: Reid G, Lynch JP 3rd, Fishbein MC, Clark NM. Mucormycosis. Semin Respir Crit Care Med. 2020 Feb;41(1):99-114. doi: 10.1055/s-0039-3401992. Epub 2020 Jan 30. PMID: 32000287.
|
| 其他信息 |
Isavuconazonium is an organic cation that is the cationic portion of isavuconazonium sulfate (a prodrug for isavuconazole, an antifungal agent used for the treatment of invasive aspergillosis and invasive mucormycosis). It has a role as a prodrug, an ergosterol biosynthesis inhibitor, an EC 1.14.13.70 (sterol 14alpha-demethylase) inhibitor and an antifungal agent.
Isavuconazonium is a second-generation triazole antifungal approved on March 6, 2015 by the FDA and July 2015 by the EMA for the treatment of adults with invasive aspergillosis and invasive mucormycosis, marketed by Astellas under the brand Cresemba. It is the prodrug form of isavuconazole, the active moiety, and it is available in oral and parenteral formulations. Due to low solubility in water of isavuconazole on its own, the isovuconazonium formulation is favorable as it has high solubility in water and allows for intravenous administration. This formulation also avoids the use of a cyclodextrin vehicle for solubilization required for intravenous administration of other antifungals such as voriconazole and posaconazole, eliminating concerns of nephrotoxicity associated with cyclodextrin. Isovuconazonium has excellent oral bioavailability, predictable pharmacokinetics, and a good safety profile, making it a reasonable alternative to its few other competitors on the market. On December 08, 2023, the FDA approved the expanded use of isovuconazonium in pediatric patients for the same indications. Isavuconazonium is a triazole antifungal agent used primarily in the treatment of invasive aspergillosis and mucormycosis infections. Isavuconazonium is associated with a low rate of transient and asymptomatic serum aminotransferase elevations during therapy, but has not been linked to instances of clinically apparent acute drug induced liver injury. Drug Indication Isavuconazonium is indicated for the treatment of invasive aspergillosis and mucormycosis in adults and pediatric patients 1 year of age and older in capsule form and adults and pediatric patients 6 years of age and older who weigh 16 kilograms (kg) and greater in injection form. FDA Label Mechanism of Action Isavuconazonium sulfate is the prodrug of isavuconazole, an azole antifungal. Isavuconazole inhibits the synthesis of ergosterol, a key component of the fungal cell membrane, by inhibiting cytochrome P-450-dependent enzyme lanosterol 14-alpha-demethylase (Erg11p). This enzyme is responsible for the conversion of lanosterol to ergosterol. An accumulation of methylated sterol precursors and a depletion of ergosterol within the fungal cell membrane weaken the membrane structure and function. Mammalian cell demethylation is less sensitive to isavuconazole inhibition. Pharmacodynamics In patients treated with isavuconazonium for invasive aspergillosis in a controlled trial, there was no significant association between plasma AUC or plasma isavuconazole concentration and efficacy. The effect on QTc interval of multiple doses of isavuconazonium capsules was evaluated. Isavuconazonium was administered as 2 capsules (equivalent to 200 mg isavuconazole) three times daily on days 1 and 2 followed by either 2 capsules or 6 capsules (equivalent to 600 mg isavuconazole) once daily for 13 days in a randomized, placebo- and active-controlled (moxifloxacin 400 mg single-dose), four-treatment-arms, parallel study in 160 healthy subjects. Isavuconazole resulted in dose-related shortening of the QTc interval. For the 2-capsule dosing regimen, the least squares mean (LSM) difference from placebo was -13.1 msec at 2 hours postdose [90% CI: -17.1, -9.1 msec]. Increasing the dose to 6 capsules resulted in an LSM difference from the placebo of -24.6 msec at 2 hours postdose [90% CI: -28.7, -20.4]. Isavuconazonium was not evaluated in combination with other drugs that reduce the QTc interval, so the additive effects are not known. The mechanism of resistance to isavuconazole, like other azole antifungals, is likely due to multiple mechanisms that include substitutions in the target gene CYP51. Changes in sterol profile and elevated efflux pump activity were observed; however, the clinical relevance of these findings is unclear. In vitro and animal studies suggest cross-resistance between isavuconazole and other azoles. The relevance of cross-resistance to clinical outcomes has not been fully characterized; however, patients failing prior azole therapy may require alternative antifungal therapy. |
| 分子式 |
C35H37F2N8O5S+
|
|---|---|
| 分子量 |
719.78
|
| 精确质量 |
717.242
|
| 元素分析 |
C, 58.40; H, 5.18; F, 5.28; N, 15.57; O, 11.11; S, 4.45
|
| CAS号 |
742049-41-8
|
| 相关CAS号 |
338990-84-4 (chloride);497235-79-7 (chloride HCl);742049-41-8 (cation);946075-13-4 (sulfate);
|
| PubChem CID |
6918606
|
| 外观&性状 |
Solid powder
|
| LogP |
4.975
|
| tPSA |
187.61
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
13
|
| 可旋转键数目(RBC) |
15
|
| 重原子数目 |
51
|
| 分子复杂度/Complexity |
1210
|
| 定义原子立体中心数目 |
2
|
| SMILES |
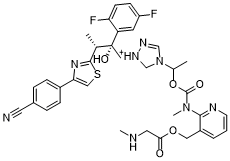
S1C=C(C2C=CC(C#N)=CC=2)N=C1[C@H](C)[C@@](C1C=C(C=CC=1F)F)(CN1C=[N+](C=N1)C(C)OC(N(C)C1C(=CC=CN=1)COC(CNC)=O)=O)O
|
| InChi Key |
AWANULZDKHTBBZ-QXLBVTBOSA-O
|
| InChi Code |
InChI=1S/C35H36F2N8O5S/c1-22(33-42-30(18-51-33)25-9-7-24(15-38)8-10-25)35(48,28-14-27(36)11-12-29(28)37)19-45-21-44(20-41-45)23(2)50-34(47)43(4)32-26(6-5-13-40-32)17-49-31(46)16-39-3/h5-14,18,20,22-23,39,48H,16-17,19,21H2,1-4H3/p+1/t22-,23?,35+/m0/s1
|
| 化学名 |
Glycine, N-methyl-, (2-(((1-(1-((2R,3R)-3-(4-(4-cyanophenyl)-2-thiazolyl)-2-(2,5-difluorophenyl)-2-hydroxybutyl)-1H-1,2,4-triazolium-4-yl)ethoxy)carbonyl)methylamino)-3-pyridinyl)methyl ester
|
| 别名 |
Isavuconazonium Free Base; BAL8557; BAL 8557; BAL-8557
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3893 mL | 6.9466 mL | 13.8931 mL | |
| 5 mM | 0.2779 mL | 1.3893 mL | 2.7786 mL | |
| 10 mM | 0.1389 mL | 0.6947 mL | 1.3893 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。