| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Mitochondrial protease OMA1
|
|---|---|
| 体外研究 (In Vitro) |
BTM化合物(如BTM-3566和BTM- 3528)在体外诱导bax依赖性DLBCL细胞死亡[1]
BTM-3566和BMT-3528诱导atf4连接的ISR激活[1] BTM-3528诱导oma1依赖性线粒体断裂[1] 与这些发现一致,删除OMA1或DELE1可以保护BJAB细胞免受BTM-3566和BTM-3528诱导的凋亡,而OPA1ΔS1/ΔS1 BJAB细胞仍然完全敏感。[1] 用BTM-3528或BTM-3566化合物处理HRI - / -和eIF2α s49a /S52A细胞后,未观察到eIF2α磷酸化和ATF4蛋白升高。[1] 线粒体蛋白FAM210B抑制BTM-3528和BTM-3566活性[1] FAM210B-tGFP表达完全抑制BTM-3528和BTM-3566的活性,但对硼替佐米或fccp诱导的细胞死亡无影响[1]。 正如预期的那样,在BTM-3528和BTM-3566存在的情况下,WT HCT-116细胞中L-OPA1发生了分裂,而BTM-3532不存在。[1] BTM-3566诱导DLBCL细胞凋亡和细胞完全杀伤,ic50为200 ~ 500 nM。应答性DLBCL细胞系包括ABC、GCB、双打和三打淋巴瘤系。[2] 转录组学和蛋白质组学分析显示,BTM-3566通过磷酸化真核翻译起始因子2α (eIF2α)和随后上调转录因子ATF4,强烈激活了ATF4整合应激反应(ISR)。在人类基因组中的四种eIF2a激酶中,我们使用crispr - cas9基因耗尽技术确定了HRI是BTM-3566 eIF2a磷酸化、诱导ATF4 ISR和细胞凋亡所唯一需要的。HRI被描述为被线粒体相关应激激活,包括血红素耗竭、ROS生成增加或线粒体ATP合成受阻,从而导致线粒体蛋白酶OMA1激活等线粒体蛋白质停滞增加。我们确定BTM-3566激活OMA1,而不像传统的线粒体毒素那样起作用。在线粒体耗氧或膜去极化未减少的情况下,BTM-3566治疗可在短短30分钟内诱导oma1依赖性的OPA1加工和线粒体断裂。这一数据表明,BTM-3566代表了一类激活线粒体蛋白酶OMA1的新化合物。[2] 基于基因表达的BTM-3566敏感性分析显示,线粒体膜蛋白FAM210B与BTM-3566的反应呈负相关。值得注意的是,在DLBCL细胞株BJAB和Burkitt淋巴瘤细胞株Ramos中,FAM210B的过表达完全阻止了OMA1的激活,并导致对btm -3566诱导的细胞凋亡的完全抵抗。因此,FAM210B可作为BTM-3566敏感性的强预测因子,并揭示了OMA1活化调节的新机制。[2] |
| 体内研究 (In Vivo) |
每日一次口服BTM-3566导致移植的人DLBCL SU-DHL-10细胞完全消退,9例DLBCL患者来源的异种移植物中有6例完全消退。BTM-3566是首个选择性激活线粒体ISR治疗DLBCL的方法。[1]
BTM-3566在人细胞系和患者来源的异种移植物模型中具有良好的药代动力学特性和有效的体内活性。为了研究BTM-3566的药代动力学特性,我们在小鼠体内进行了静脉/口服交叉研究(图2A-C)。生物利用度>90%,每日口服一次的终末半衰期为4.4 ~ 6.6小时是可以接受的[1]。 BTM-3566的治疗活性在使用双打淋巴瘤DLBCL肿瘤系SU-DHL-10的人类异种移植模型中进行了评估(图2D)。在10 mg/kg剂量下,我们观察到肿瘤生长延迟,随着进一步给药而消失(图2E,顶部)。在剂量等于或高于20mg /kg时,BTM-3566治疗导致完全缓解(CR;定义为在给药10天后所有动物均无可触及的肿瘤,并维持给药21天。为了评估BTM治疗是否会引起持久的反应,动物在停止给药后再随访30天。BTM-3566剂量为20 mg/kg的动物中有40%的动物和30 mg/kg的动物保持了30天无肿瘤生存(图2E顶部)。体重减轻是剂量依赖性的,但在20 mpk剂量水平下体重减轻<10%(图2E底部)。在30 mg/kg剂量组,10只小鼠中有2只体重减轻超过20%,需要计划外的给药假期。两组的体重减轻在停止给药后都是可逆的。(图2E底部).[1] 在SU-DHL10模型中确定20mg /kg剂量有效且耐受性良好后,我们接下来在9个人类DLBCL患者来源的异种移植(PDX)模型中测试了BTM-3566,这些模型代表了具有高风险基因型的ABC和GCB DLBCL亚型(图2F- - h)。用20 mg/kg BTM-3566治疗9个PDX模型中的6个模型中的3只小鼠均出现CR。将9种模型治疗组27只小鼠合组,66%(19/27)小鼠出现CR,另有4只小鼠出现部分缓解(PR), 2只小鼠肿瘤稳定,2只小鼠病情进展。总之,单药总有效率(CR + PR)为85.2%,所有模型至少有一只动物表现出完全或部分回归(图2H)。[1] 复发/难治性弥漫性大b细胞淋巴瘤(r/r- dlbcl)是一个治疗挑战,特别是对于不适合大剂量化疗、干细胞移植或car - t细胞治疗失败的患者。r/r- dlbcl在临床和分子上都是高度异质性的,这就迫切需要开发新的治疗方法来改善独立于分子亚型的患者的预后。我们在这里描述了BTM-3566,一种具有抗多种b细胞恶性肿瘤活性的一流化合物,但对DLBCL的作用最大。BTM-3566通过线粒体蛋白FAM210B调控的新机制激活线粒体综合应激反应(ISR)。BTM-3566在体外诱导DLBCL细胞系凋亡,在体内诱导预后不良的基因改变的DLBCL PDX小鼠模型完全肿瘤消退。[2] BTM-3566是一种基于吡唑噻唑骨架的口服小分子,用于治疗弥漫性大b细胞淋巴瘤(DLBCL)。小鼠的药代动力学研究表明,每天给药一次,具有> 50%的口服生物利用度和接近6小时的血清半衰期。小鼠和犬的14天剂量在治疗剂量下表现出良好的耐受性。BTM-3566在人肝细胞中表现出稳定性(IC < 5 ml/min*kg),并且具有良好的体外安全性。在使用双靶向DLBCL系SU-DHL-10的异种移植模型中,BTM-3566治疗在所有荷瘤动物中均导致完全消退。最重要的是,在停止治疗后的2周内没有肿瘤生长,这表明BTM-3566治疗导致该模型的双重打击DLBCL持久完全缓解。对包含一系列高风险基因组改变(包括Myd88突变和MYC和BCL2重排)的人类DLBCL PDX模型的扩展研究表明,在8个PDX模型中,有6个模型的100%的品系肿瘤完全消退(表1)。[2] |
| 酶活实验 |
半胱天冬酶分析[1]< br >
用BTM-3528或BTM-3566处理细胞后,检测Caspase 3/7活性。使用Caspase-Glo®3/7检测。所有细胞用化合物处理24小时,然后按照制造商的说明处理Caspase活性。[1]
图像分析[1] 使用Fiji/ImageJ软件和可训练的Weka分割插件评估线粒体形态。根据MTG通道获得的分节线粒体结构计算线粒体膜电位,TMRE/MTG荧光比。统计学分析采用单因素方差分析和Tukey多重比较检验;P值≤0.05(*)认为差异有统计学意义[1]。 线粒体呼吸运动计量法[1] 在海马细胞外通量分析仪上进行呼吸测定。HCT-116细胞使用XF96孔微孔板以14000个细胞/孔接种,在含有10%胎牛血清的McCoy's 5a Modified Medium培养基中(37°C, 5% CO2)孵育过夜。在进行呼吸测定前,用含10 mmol/L葡萄糖、2 mmol/L谷氨酰胺、1 mmol/L丙酮酸、5 mmol/L HEPES和10%胎牛血清(pH 7.4)的DMEM培养液洗涤细胞。以3 μmol/L的终浓度检测BTM化合物,在检测过程中对细胞进行急性处理或在检测前预处理4小时。在预处理实验中,化合物加入到完全培养基中,在37°C和5% CO2下孵育。实验中注射的化合物为寡霉素2 μmol/L, FCCP 1 μmol/L,抗霉素A和鱼藤酮2 μmol/L。每次呼吸测定结束后,用1 μg/mL Hoechst染色细胞,用Operetta高含量成像系统测定细胞数量。每个实验的呼吸水平数据(pmol O2/min)归一化为每孔细胞数(pmol O2/min/103个细胞)。 |
| 细胞实验 |
细胞系复合试验[1]
肿瘤细胞系的筛选由Crown Bioscience完成。以4 × 103个细胞/孔的初始密度镀细胞,孵育24小时。BTM-3528为被试品的10倍溶液,最终工作浓度为30 μmol/L,在9次3.16倍连续稀释的培养基中制备。加入BTM-3528后,在37℃、5% CO2条件下再孵育96小时。使用cell - titre Glo法测定最终细胞数。绝对IC50曲线采用具有s型剂量响应的非线性回归模型拟合。每个化合物的活性区(AUC)是通过计算每个剂量反应曲线拟合的综合面积界来确定的。AUC反映了作用的大小(最大抑制)和效价(IC50)。[1] 膜联蛋白V凋亡试验[1] 为了量化细胞凋亡,我们使用含有15%胎牛血清的RPMI培养液培养BJAB,并在指定的时间间隔内用BTM化合物处理。用冷冻PBS洗涤细胞2次,用浓度为1× 106个细胞/ml的1× Binding Buffer重悬。取0.5 × 105个细胞,加入2.5 μL Annexin V-APC或Annexin V-FITC,室温黑暗孵育15分钟。用结合缓冲液冲洗细胞一次,将微球重悬于含有2 μL碘化丙啶(50 μg/mL)或2 μL DAPI (1 mg/mL)的100 μL结合缓冲液中。在Cytoflex s上分析细胞。[1] 转录组分析[1]< br > 以人结肠癌细胞株HCT-116为实验材料,研究BTM化合物对基因表达的影响。为了充分评估化合物对细胞周期控制基因的影响,在BTM化合物处理之前,细胞被同步。在添加10%胎牛血清和青霉素/链霉素的McCoys 5a培养基中生长的HCT-116细胞首先通过胸苷阻断s期。24小时后,去除含胸苷嘧啶的培养基,用含诺可唑的培养基替代,以高度同步地阻断m期细胞。然后将细胞在完全培养液或完全培养液中添加10、1或0.1 μmol/L BTM-3528释放到G1中。在释放至G1期后的1、2、4、6和8小时,分别收集细胞,提取mRNA进行Illumina RNA测序(RNA-seq)。收集每个浓度和时间点的三个重复以及特定时间点的对照(即无化合物的细胞)进行RNA-seq。 |
| 动物实验 |
In vivo efficacy of BTM-3566[1]
Human cell line xenograft models were established using SU-DHL-10 cells. Cells were grown in RPMI1640 supplemented with 15% fetal bovine serum and penicillin/streptomycin. Cells were harvested by centrifugation and resuspended in cold 50% serum-free medium: 50% Matrigel to generate a final concentration of 2.50E+07 trypan-excluding cells/mL. Female Envigo SCID beige mice (C.B-17/IcrHsd Prkdcscidlystbg-j) were implanted subcutaneously high in the right axilla on day 0 with 5 × 106 cells/mouse. Mice were randomized into groups based on tumor volume with a mean tumor burden for each group of 150 mm3. BTM-3566 was prepared as a solution in dosing vehicle containing 5% NMP, 15% PEG400, 10% Solutol, and 70% D5W. The final dose concentration was 4 mg/mL, and the dose volume was 5 μL/gram. All mice were dosed by oral gavage once daily for 21 days. Tumor volume and body weights were determined every third day. All mice were dosed according to individual body weight on the day of treatment.[1] For patient derived xenograft models, all tumors were sourced from Crown Bio. Models were established in female mice with an average body weight of 25 grams. Balb/c nude,NOD SCID mice, or NPG/NOD/SCID were used. Each mouse was inoculated subcutaneously in the right flank region with fresh tumor derived from mice bearing established primary human cancer tissue. Mice were randomized into vehicle or treatment groups with a mean tumor burden of 200 mm3. All mice were dosed once daily by oral gavage for 21 days. Tumor volume and body weights were determined three times per week. [1] Pharmacokinetic Analysis of BTM-3566 in mouse blood[1] Blood was collected from a tail vein snip into K2-EDTA tubes. Plasma was isolated and a 20 mL sample was protein precipitated with 200 L of acetonitrile containing 100 ng/mL diclofenac, tolbutamide and labetalol as internal standards. The mixture was vortex-mixed and centrifuged at 13000 rpm for 15 min, 4 ℃. An 80 mL aliquot of the supernatant was transferred to a sample plate and mixed with 80 μL water, then the plate was shaken at 800 rpm for 10 min. A 1 L aliquot was injected on to a Waters ACQUITY UPLC BEH C18 2.1*50mm, 1.7µm reverse phase column using a two-component mobile phase gradient. Mobile phase A was 0.1% trifluoracetic acid in water and mobile phase B 0.1% TFA in acetonitrile. Plasma BTM-3566 was detected using electrospray ionization and multiple reaction monitoring mass spectrometry (BTM-3528 [M+H]+ m/z 522.10>203.1. All data were analyzed using single compartment analysis in WinNonLin.[1] |
| 药代性质 (ADME/PK) |
To investigate the pharmacokinetic properties of BTM-3566, researchers performed intravenous/oral crossover studies in mice. Bioavailability was >90% and the terminal half-life of 4.4 to 6.6 hours was acceptable for oral, once daily dosing.[1]
|
| 参考文献 |
|
| 其他信息 |
DLBCL are aggressive, rapidly proliferating tumors that critically depend on the ATF4-mediated integrated stress response (ISR) to adapt to stress caused by uncontrolled growth, such as hypoxia, amino acid deprivation, and accumulation of misfolded proteins. Here, we show that ISR hyperactivation is a targetable liability in DLBCL. We describe a novel class of compounds represented by BTM-3528 and BTM-3566, which activate the ISR through the mitochondrial protease OMA1. Treatment of tumor cells with compound leads to OMA1-dependent cleavage of DELE1 and OPA1, mitochondrial fragmentation, activation of the eIF2α-kinase HRI, cell growth arrest, and apoptosis. Activation of OMA1 by BTM-3528 and BTM-3566 is mechanistically distinct from inhibitors of mitochondrial electron transport, as the compounds induce OMA1 activity in the absence of acute changes in respiration. We further identify the mitochondrial protein FAM210B as a negative regulator of BTM-3528 and BTM-3566 activity. Overexpression of FAM210B prevents both OMA1 activation and apoptosis. Notably, FAM210B expression is nearly absent in healthy germinal center B-lymphocytes and in derived B-cell malignancies, revealing a fundamental molecular vulnerability which is targeted by BTM compounds. Both compounds induce rapid apoptosis across diverse DLBCL lines derived from activated B-cell, germinal center B-cell, and MYC-rearranged lymphomas. Once-daily oral dosing of BTM-3566 resulted in complete regression of xenografted human DLBCL SU-DHL-10 cells and complete regression in 6 of 9 DLBCL patient-derived xenografts. BTM-3566 represents a first-of-its kind approach of selectively hyperactivating the mitochondrial ISR for treating DLBCL.[1]
Relapsed/refractory diffuse large B-cell lymphomas (r/r-DLBCL) are a therapeutic challenge, especially in patients not suitable for high dose chemotherapy, stem cell transplantation or patients who fail CAR-T-cell therapy. r/r-DLBCLs are highly heterogeneous both clinically and molecularly, which imposes a pressing need to develop novel therapies to improve outcomes in patients independently of the molecular subtype. We describe here BTM-3566, a first-in-class compound with activity against a variety of B-cell malignancies but with greatest effect in DLBCL. BTM-3566 activates the mitochondrial integrated stress response (ISR) through a novel mechanism regulated by the mitochondrial protein FAM210B. BTM-3566 induces apoptosis in DLBCL lines in vitro and complete tumor regression in vivo in DLBCL PDX mouse models harboring genetic alterations associated with poor prognosis.[2] BTM-3566 is an oral small molecule based on a pyrazolothiazol-backbone, developed for treatment of diffuse large B-cell lymphoma (DLBCL). BTM-3566 induces apoptosis and complete cell killing in DLBCL lines a with an IC 50 of ~200 - 500 nM. Responsive DLBCL cell lines include ABC, GCB, and double-hit and triple-hit lymphoma lines. Pharmacokinetic studies in mice showed suitability for once daily dosing, with > 50% of oral bioavailability and close to 6 hours of serum half-life. 14-day dosing in mice and dogs demonstrated excellent tolerability at therapeutic doses. BTM-3566 showed stability in human hepatocytes (IC < 5 ml/min*kg) as well and a favorable in vitro safety profile. In xenograft models using the double-hit DLBCL line SU-DHL-10, BTM-3566 treatment resulted in complete regression in all tumor-bearing animals. Most importantly, no subsequent tumor growth occurred for 2 weeks after cessation of therapy, indicating that treatment with BTM-3566 resulted in a durable complete remission in this model of double-hit DLBCL. Expansion studies into human DLBCL PDX models harboring a range of high-risk genomic alterations, including Myd88 mutations and MYC and BCL2 rearrangements, demonstrated response in 100% of the lines with complete tumor regression in 6 of 8 PDX models tested (Table 1).[2] Transcriptome and proteome analyses revealed that BTM-3566 strongly activated the ATF4-integrated stress response (ISR), indicated by phosphorylation of eukaryotic translation initiation factor 2α (eIF2α) and subsequent upregulation of the transcription factor ATF4. Of the four eIF2a-kinases in the human genome we determined, using CRISPR-Cas 9 gene depletion, that HRI was uniquely required for BTM-3566 eIF2a phosphorylation, induction of ATF4 ISR and apoptosis. HRI is described as being activated by mitochondrial-related stress, including heme depletion, increased ROS generation or blockage of mitochondrial ATP synthesis which result in an increase in mitochondrial proteostasis including activation of mitochondrial protease OMA1. We determined that BTM-3566 activates OMA1 without acting as a classical mitochondrial toxin. Treatment with BTM-3566 induced OMA1-dependent OPA1 processing and mitochondrial fragmentation in as little as 30 minutes of treatment, in the absence of any reduction in mitochondrial oxygen consumption or membrane depolarization. This data indicates that BTM-3566 represents a new class of compounds that activate the mitochondrial protease OMA1.[2] Gene expression-based profiling of BTM-3566 sensitivity in over 400 cancer cell lines showed that FAM210B, a mitochondrial membrane protein, negatively correlated with response to BTM-3566. Notably, overexpression of FAM210B completely prevents OMA1 activation and causes complete resistance to BTM-3566-induced apoptosis in DLBCL cell line BJAB and the Burkitt lymphoma cell line Ramos. Thus, FAM210B serves as a strong predictor of BTM-3566 sensitivity, as well as revealing a novel mechanism of regulation of OMA1 activation.[2] In summary, we describe here a novel, highly potent activator of the mitochondrial ISR, which is well tolerated in mice and dogs, has favorable pharmacokinetics and induces robust DLBCL regression in-vivo. An IND application in B-cell malignancies will be completed by early Q1 2022 with initiation of first in human clinical trials the first half of 2022.[2] |
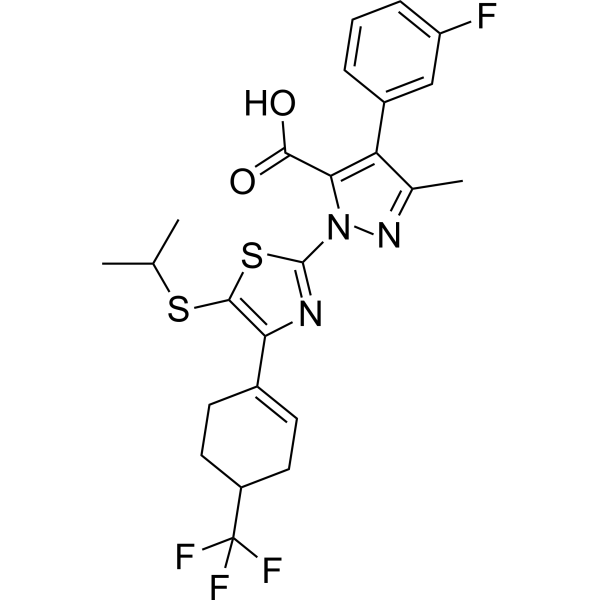
| 分子式 |
C24H23F4N3O2S2
|
|---|---|
| 分子量 |
525.581937074661
|
| 精确质量 |
525.116
|
| CAS号 |
2228857-70-1
|
| PubChem CID |
134564305
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| LogP |
7.3
|
| tPSA |
122Ų
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
35
|
| 分子复杂度/Complexity |
799
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
LDSVVHIOWFIJNE-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C24H23F4N3O2S2/c1-12(2)34-22-19(14-7-9-16(10-8-14)24(26,27)28)29-23(35-22)31-20(21(32)33)18(13(3)30-31)15-5-4-6-17(25)11-15/h4-7,11-12,16H,8-10H2,1-3H3,(H,32,33)
|
| 化学名 |
4-(3-fluorophenyl)-5-methyl-2-[5-propan-2-ylsulfanyl-4-[4-(trifluoromethyl)cyclohexen-1-yl]-1,3-thiazol-2-yl]pyrazole-3-carboxylic acid
|
| 别名 |
BTM-3566; 2228857-70-1; BTM3566; SCHEMBL20214005; LDSVVHIOWFIJNE-UHFFFAOYSA-N;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9027 mL | 9.5133 mL | 19.0266 mL | |
| 5 mM | 0.3805 mL | 1.9027 mL | 3.8053 mL | |
| 10 mM | 0.1903 mL | 0.9513 mL | 1.9027 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。